Lithium diisopropylamide on:
[Wikipedia]
[Google]
[Amazon]
Lithium diisopropylamide (commonly abbreviated LDA) is a
 LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of
LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of MSDS
at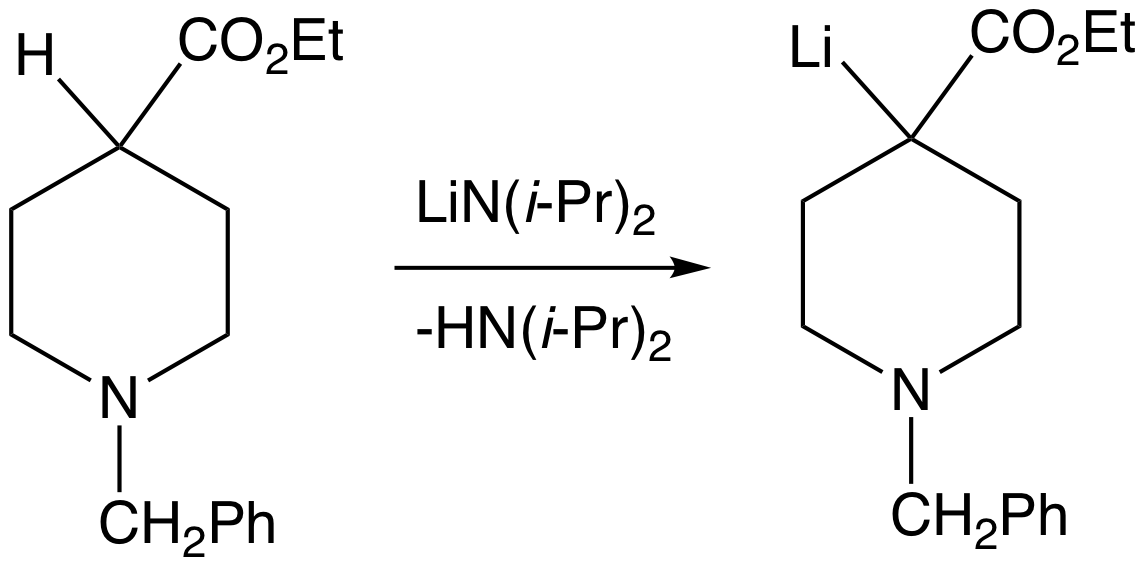
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the molecular formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
. It is used as a strong base and has been widely utilized due to its good solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
in non-polar organic solvents and non-nucleophilic nature. It is a colorless solid, but is usually generated and observed only in solution. It was first prepared by Hamell and Levine in 1950 along with several other hindered lithium diorganylamides to effect the deprotonation of esters at the α position without attack of the carbonyl group.
Preparation and structure
 LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of
LDA is commonly formed by treating a cooled (0 to −78 °C) mixture of tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
and diisopropylamine with ''n''-butyllithium.
When dissociated, the diisopropylamide anion can become protonated
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, ...
to form diisopropylamine. Diisopropylamine has a p''K''a value of 36. Therefore, its conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
is suitable for the deprotonation of compounds with greater acidity, importantly, such weakly acidic compounds (carbon acids) of the type , where Z = C(O)R', C(O)OR' or CN. Conventional protic functional groups such as alcohols and carboxylic acids are readily deprotonated.
Like most organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
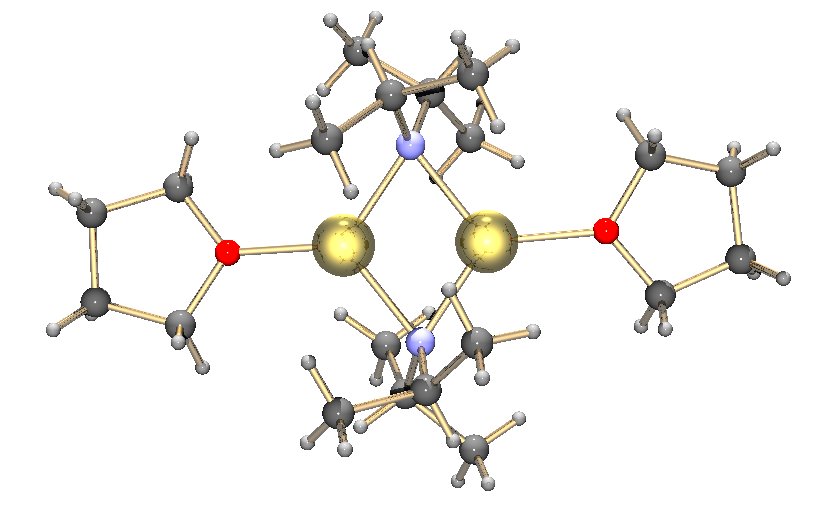
s, LDA is not a salt, but is highly polar. It forms aggregates in solution, with the extent of aggregation depending on the nature of the solvent. In THF its structure is primarily that of a solvated dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ...
. In nonpolar solvents such as toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
, it forms a temperature-dependent oligomer equilibrium. At room temperature trimers and tetramers are the most likely structures. With decreasing temperature the aggregation extends to pentameric and higher oligomeric structures.
Solid LDA is pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
,at
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group.
Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company a ...
but its solutions are generally not. As such it is commercially available as a solution in polar aprotic solvents such as THF and ether; however, for small scale use (less than 50 mmol), it is common and more cost effective to prepare LDA ''in situ''.

Kinetic vs thermodynamic bases
The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction control. Kinetic controlled deprotonation requires a base that is sterically hindered and strong enough to remove the proton irreversibly. For example, in the case ofphenylacetone
Phenylacetone is an organic compound with the chemical formula C6H5CH2COCH3. It is a colorless oil that is soluble in organic solvents. This substance is used in the manufacture of methamphetamine and amphetamine, where it is commonly known a ...
, deprotonation can produce two different enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
s. LDA has been shown to deprotonate the methyl group, which is the kinetic course of the deprotonation. To ensure the production of the kinetic product, a slight excess (1.1 equiv) of lithium diisopropylamide is used, and the ketone is added to the base at –78 °C. Because the ketone is quickly and quantitatively converted to the enolate and base is present in excess at all times, the ketone is unable to act as a proton shuttle to catalyze the gradual formation of the thermodynamic product. A weaker base such as an alkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
, which reversibly deprotonates the substrate, affords the more thermodynamically stable benzylic enolate. An alternative to the weaker base is to use a strong base which is present at a lower concentration than the ketone. For instance, with a slurry
A slurry is a mixture of denser solids suspended in liquid, usually water. The most common use of slurry is as a means of transporting solids or separating minerals, the liquid being a carrier that is pumped on a device such as a centrifugal pu ...
of sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in co ...
in THF or dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majo ...
(DMF), the base only reacts at the solution-solid interface. A ketone molecule might be deprotonated at the ''kinetic'' site. This enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electr ...
may then encounter other ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s and the thermodynamic enolate will form through the exchange of protons, even in an aprotic solvent which does not contain hydronium ions.
LDA can, however, act as a nucleophile under certain conditions.
See also
*Lithium amide
Lithium amide or lithium azanide is an inorganic compound with the chemical formula . It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:
:
Other lithium amides
The co ...
*Lithium bis(trimethylsilyl)amide
Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula . It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong ...
(LiHMDS)
*Lithium tetramethylpiperidide
Lithium tetramethylpiperidide (often abbreviated LiTMP or LTMP) is a chemical compound with the molecular formula . It is used as a non-nucleophilic base, being comparable to LiHMDS in terms of steric hindrance.
Synthesis
It is synthesised by the ...
(LiTMP)
References
{{Lithium compounds Lithium compounds Diisopropylamino compounds Organolithium compounds Metal amides Non-nucleophilic bases Reagents for organic chemistry Superbases