|
Metal Amides
Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amide ligands have two electron pairs available for bonding. In principle, they can be terminal or bridging. In these two examples, the dimethylamido ligands are both bridging and terminal: File:Tris(dimethylamino)aluminium dimer.png, Tris(dimethylamino)aluminium dimer File:Tris(dimethylamino)gallium dimer.png, Tris(dimethylamino)gallium dimer File:Ti(NMe2)4.png, Tetrakis(dimethylamino)titanium File:Ta(NMe2)5.png, Pentakis(dimethylamido)tantalum In practice, bulky amide ligands have a lesser tendency to bridge. Amide ligands may participate in metal-ligand π-bonding giving a complex with the metal center being co-planar with the nitrogen and substituents. Metal bis(trimethylsilyl)amides form a significant subcategory of metal amide compounds. These compounds tend to be discrete and soluble in organic solvents. Alkali metal amid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Imide
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−). In addition to solid state imides, molecular imides are also known in dilute gases, where their spectrum can be studied. Imide can be a ligand, with a double bond to a metal such as molybdenum (e.g. Mo=NH). As a ligand it is called imido. The imido ligand is part of a nitrogen fixation cycle: Mo•N2 → Mo-N=N− → Mo-N=NH (diazenido) → Mo-N=NH2+ → Mo=N-NH2 (hydrazido) → Mo=N-NH3+ (hydrazidium) → Mo≡N (nitrido) + NH3 → Mo≡NH+ → Mo=NH (imido) → Mo=NH2+ → Mo-NH2 (amido) → Mo-NH3+ → Mo•NH3 (ammine); with the oxidation state of molybdenum varying to accommodate the number bonds from nitrogen. When the hydrogen of the imide group is sub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
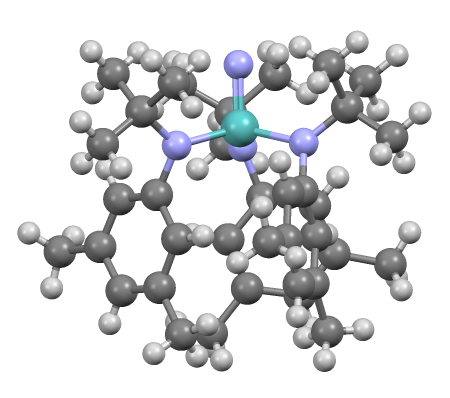
Chloropentamminecobalt Chloride
Chloropentamminecobalt chloride is the dichloride salt of the coordination complex o(NH3)5Clsup>2+. It is a red-violet, diamagnetic, water-soluble salt. The compound has been of academic and historical interest. Synthesis and reactions The salt is prepared with a two-step process starting with oxidizing a solution of cobalt chloride and ammonia. :2 CoCl2·6H2O + 10 NH3 + 2 HCl + H2O2 → 2 o(NH3)5(OH2)l3 + 12 H2O This intermediate is then heated to induce coordination of one of the outer sphere chloride ligands: : o(NH3)5(OH2)l3 → o(NH3)5Cll2 + H2O The dication o(NH3)5Clsup>2+ has idealized C4v symmetry. In an aqueous solution, chloropentaamminecobalt(III) chloride reforms aquopentammine complex. With concentrated sulfuric acid, chloropentaamminecobalt(III) chloride forms the hydrogen sulfate complex o(NH3)5OSO3Hsup>2+. History Cobalt complexes have been of long-standing interest in inorganic chemistry because they are numerous, easily prepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Ammine Complex
In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia () ligand. "Ammine" is spelled this way due to historical reasons; in contrast, alkyl or aryl bearing ligands are spelt with a single "m". Almost all metal ions bind ammonia as a ligand, but the most prevalent examples of ammine complexes are for Cr(III), Co(III), Ni(II), Cu(II) as well as several platinum group metals.A. von Zelewsky "Stereochemistry of Coordination Compounds" John Wiley: Chichester, 1995. . History Ammine complexes played a major role in the development of coordination chemistry, specifically determination of the stereochemistry and structure. They are easily prepared, and the metal-nitrogen ratio can be determined by elemental analysis. Through studies mainly on the ammine complexes, Alfred Werner developed his Nobel Prize-winning concept of the structure of coordination compounds (see Figure). One of the first ammine complexes to be described was Magnus' green ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide Complex
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, such as palladium chloride. File:NaCl polyhedra.png, Sodium chloride crystal structure File:Uranium-hexafluoride-unit-cell-3D-balls.png, Discrete UF6 molecules File:Alpha-palladium(II)-chloride-xtal-3D-balls.png, Infinite chains of one form of palladium chloride Preparation The halogens can all react with metals to form metal halides according to the following equation: :2M + nX2 → 2MXn where M is the metal, X is the halogen, and MXn is the metal halide. In practice, this type of reaction may be very exothermic, hence impractical as a preparative technique. Additionally, many transition metals can adopt multiple oxidation states, which complicates matters. As the halogens are strong oxidizers, direct combination of the elements usua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisopropylamine
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent. Reactions and use Diisopropylamine is a common amine nucleophile in organic synthesis. Because it is bulky, it is a more selective nucleophile than, say, dimethylamine. It reacts with organolithium reagents to give lithium diisopropylamide (LDA). LDA is a strong, non-nucleophilic base The main commercial applications of diisopropylamine is as a precursor to two herbicides, diallate and triallate, as well as certain sulfenamides used in the vulcanization of rubber. It is also used to prepare N,N-Diisopropylethylamine (Hünig's base) by alkylation with diethyl sulfate. The bromide salt of diisopropylamine, diisopropylammonium bromide, is a room-temperature organic ferroelectric material. Preparation Diisopropylamine, which is comme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-nucleophilic Base
As the name suggests, a non-nucleophilic base is a sterically hindered organic base that is a poor nucleophile. Normal bases are also nucleophiles, but often chemists seek the proton-removing ability of a base without any other functions. Typical non-nucleophilic bases are bulky, such that protons can attach to the basic center but alkylation and complexation is inhibited. Non-nucleophilic bases A variety of amines and nitrogen heterocycles are useful bases of moderate strength (pKa of conjugate acid * ''N'',''N''-Diisopropylethylamine (DIPEA, also called Hünig's Base), p *1,8-Diazabicycloundec-7-ene (DBU) - useful for E2 elimination reactions, pKa = 13.5 * 1,5-Diazabicyclo(4.3.0)non-5-ene (DBN) - comparable to DBU * 2,6-Di-tert-butylpyridine, a weak non-nucleophilic base pKa = 3.58 * Phosphazene bases, such as t-Bu-P4''Activation in anionic polymerization: Why phosphazene bases are very exciting promoters'' S. Boileau, N. Illy Prog. Polym. Sci., 2011, 36, 1132-1151, {{doi, 10. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

-3D-balls.png)
-3D-balls.png)

