Lanthanum-140 on:
[Wikipedia]
[Google]
[Amazon]
 Naturally occurring
Naturally occurring
 Naturally occurring
Naturally occurring lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lant ...
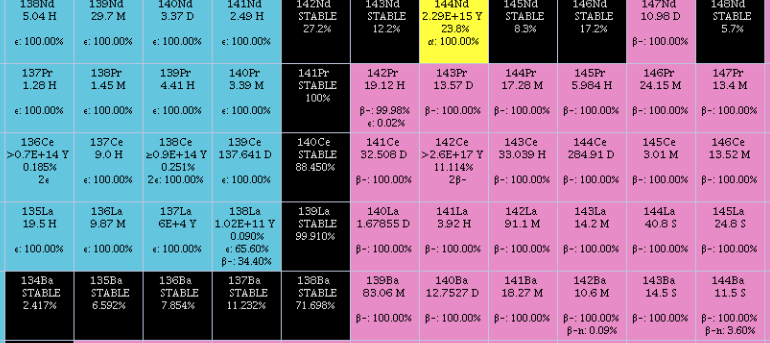
(57La) is composed of one stable (139La) and one radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
(138La) isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numb ...
, with the stable isotope, 139La, being the most abundant (99.91% natural abundance
In physics, natural abundance (NA) refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass (a weighted average, weighted by mole-fraction abundance figures) of these isotopes is the atomi ...
). There are 38 radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s that have been characterized, with the most stable being 138La, with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable at ...
of 1.02×1011 years; 137La, with a half-life of 60,000 years and 140La, with a half-life of 1.6781 days. The remaining radioactive isotopes have half-lives that are less than a day and the majority of these have half-lives that are less than 1 minute. This element also has 12 nuclear isomer
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ...
s, the longest-lived of which is 132mLa, with a half-life of 24.3 minutes.
The isotopes of lanthanum range in atomic weight
Relative atomic mass (symbol: ''A''; sometimes abbreviated RAM or r.a.m.), also known by the deprecated synonym atomic weight, is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a giv ...
from 116.95 u (117La) to 154.96 u (155La).
List of isotopes
, - , rowspan=2, 117La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 60 , rowspan=2, 116.95007(43)# , rowspan=2, 23.5(26) ms , β+ , 117Ba , rowspan=2, (3/2+, 3/2−) , rowspan=2, , rowspan=2, , - , p , 116Ba , - , style="text-indent:1em" , 117mLa , colspan="3" style="text-indent:2em" , 151(12) keV , 10(5) ms , , , (9/2+) , , , - , 118La , style="text-align:right" , 57 , style="text-align:right" , 61 , 117.94673(32)# , 200# ms , β+ , 118Ba , , , , - , 119La , style="text-align:right" , 57 , style="text-align:right" , 62 , 118.94099(43)# , 1# s , β+ , 119Ba , 11/2−# , , , - , rowspan=2, 120La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 63 , rowspan=2, 119.93807(54)# , rowspan=2, 2.8(2) s , β+ , 120Ba , rowspan=2, , rowspan=2, , rowspan=2, , - , β+, p , 119Cs , - , rowspan=2, 121La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 64 , rowspan=2, 120.93301(54)# , rowspan=2, 5.3(2) s , β+ , 121Ba , rowspan=2, 11/2−# , rowspan=2, , rowspan=2, , - , β+, p , 120Cs , - , rowspan=2, 122La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 65 , rowspan=2, 121.93071(32)# , rowspan=2, 8.6(5) s , β+ , 122Ba , rowspan=2, , rowspan=2, , rowspan=2, , - , β+, p , 121Cs , - , 123La , style="text-align:right" , 57 , style="text-align:right" , 66 , 122.92624(21)# , 17(3) s , β+ , 123Ba , 11/2−# , , , - , 124La , style="text-align:right" , 57 , style="text-align:right" , 67 , 123.92457(6) , 29.21(17) s , β+ , 124Ba , (7−, 8−) , , , - , style="text-indent:1em" , 124mLa , colspan="3" style="text-indent:2em" , 100(100)# keV , 21(4) s , , , low(+#) , , , - , 125La , style="text-align:right" , 57 , style="text-align:right" , 68 , 124.920816(28) , 64.8(12) s , β+ , 125Ba , (11/2−) , , , - , style="text-indent:1em" , 125mLa , colspan="3" style="text-indent:2em" , 107.0(10) keV , 390(40) ms , , , (3/2+) , , , - , 126La , style="text-align:right" , 57 , style="text-align:right" , 69 , 125.91951(10) , 54(2) s , β+ , 126Ba , (5)(+#) , , , - , style="text-indent:1em" , 126mLa , colspan="3" style="text-indent:2em" , 210(410) keV , 20(20) s , , , (0−, 1−, 2−) , , , - , 127La , style="text-align:right" , 57 , style="text-align:right" , 70 , 126.916375(28) , 5.1(1) min , β+ , 127Ba , (11/2−) , , , - , rowspan=2 style="text-indent:1em" , 127mLa , rowspan=2 colspan="3" style="text-indent:2em" , 14.8(12) keV , rowspan=2, 3.7(4) min , β+ , 127Ba , rowspan=2, (3/2+) , rowspan=2, , rowspan=2, , - , IT , 127La , - , 128La , style="text-align:right" , 57 , style="text-align:right" , 71 , 127.91559(6) , 5.18(14) min , β+ , 128Ba , (5+) , , , - , style="text-indent:1em" , 128mLa , colspan="3" style="text-indent:2em" , 100(100)# keV , <1.4 min , IT , 128La , (1+, 2−) , , , - , 129La , style="text-align:right" , 57 , style="text-align:right" , 72 , 128.912693(22) , 11.6(2) min , β+ , 129Ba , 3/2+ , , , - , style="text-indent:1em" , 129mLa , colspan="3" style="text-indent:2em" , 172.1(4) keV , 560(50) ms , IT , 129La , 11/2− , , , - , 130La , style="text-align:right" , 57 , style="text-align:right" , 73 , 129.912369(28) , 8.7(1) min , β+ , ''130Ba'' , 3(+) , , , - , 131La , style="text-align:right" , 57 , style="text-align:right" , 74 , 130.91007(3) , 59(2) min , β+ , 131Ba , 3/2+ , , , - , style="text-indent:1em" , 131mLa , colspan="3" style="text-indent:2em" , 304.52(24) keV , 170(10) μs , , , 11/2− , , , - , 132La , style="text-align:right" , 57 , style="text-align:right" , 75 , 131.91010(4) , 4.8(2) h , β+ , 132Ba , 2− , , , - , rowspan=2 style="text-indent:1em" , 132mLa , rowspan=2 colspan="3" style="text-indent:2em" , 188.18(11) keV , rowspan=2, 24.3(5) min , IT (76%) , 132La , rowspan=2, 6− , rowspan=2, , rowspan=2, , - , β+ (24%) , 132Ba , - , 133La , style="text-align:right" , 57 , style="text-align:right" , 76 , 132.90822(3) , 3.912(8) h , β+ , 133Ba , 5/2+ , , , - , 134La , style="text-align:right" , 57 , style="text-align:right" , 77 , 133.908514(21) , 6.45(16) min , β+ , 134Ba , 1+ , , , - , 135La , style="text-align:right" , 57 , style="text-align:right" , 78 , 134.906977(11) , 19.5(2) h , β+ , 135Ba , 5/2+ , , , - , 136La , style="text-align:right" , 57 , style="text-align:right" , 79 , 135.90764(6) , 9.87(3) min , β+ , 136Ba , 1+ , , , - , style="text-indent:1em" , 136mLa , colspan="3" style="text-indent:2em" , 255(9) keV , 114(3) ms , IT , 136La , (8)(−#) , , , - , 137La , style="text-align:right" , 57 , style="text-align:right" , 80 , 136.906494(14) , 6(2)×104 y , EC , 137Ba , 7/2+ , , , extinct , - , rowspan=2, 138La Primordial radionuclide , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 81 , rowspan=2, 137.907112(4) , rowspan=2, 1.02(1)×1011 y , β+ (66.4%) , 138Ba , rowspan=2, 5+ , rowspan=2, 9.0(1)×10−4 , rowspan=2, , - , β− (33.6%) , 138Ce , - , style="text-indent:1em" , 138mLa , colspan="3" style="text-indent:2em" , 72.57(3) keV , 116(5) ns , , , (3)+ , , , - , 139La Fission product , style="text-align:right" , 57 , style="text-align:right" , 82 , 138.9063533(26) , colspan=3 align=center, StableTheoretically capable of spontaneous fission , 7/2+ , 0.99910(1) , , - , 140La , style="text-align:right" , 57 , style="text-align:right" , 83 , 139.9094776(26) , 1.6781(3) d , β− , 140Ce , 3− , , , - , 141La , style="text-align:right" , 57 , style="text-align:right" , 84 , 140.910962(5) , 3.92(3) h , β− , 141Ce , (7/2+) , , , - , 142La , style="text-align:right" , 57 , style="text-align:right" , 85 , 141.914079(6) , 91.1(5) min , β− , 142Ce , 2− , , , - , 143La , style="text-align:right" , 57 , style="text-align:right" , 86 , 142.916063(17) , 14.2(1) min , β− , 143Ce , (7/2)+ , , , - , 144La , style="text-align:right" , 57 , style="text-align:right" , 87 , 143.91960(5) , 40.8(4) s , β− , 144Ce , (3−) , , , - , 145La , style="text-align:right" , 57 , style="text-align:right" , 88 , 144.92165(10) , 24.8(20) s , β− , 145Ce , (5/2+) , , , - , rowspan=2, 146La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 89 , rowspan=2, 145.92579(8) , rowspan=2, 6.27(10) s , β− (99.99%) , 146Ce , rowspan=2, 2− , rowspan=2, , rowspan=2, , - , β−, n (.007%) , 145Ce , - , style="text-indent:1em" , 146mLa , colspan="3" style="text-indent:2em" , 130(130) keV , 10.0(1) s , β− , 146Ce , (6−) , , , - , rowspan=2, 147La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 90 , rowspan=2, 146.92824(5) , rowspan=2, 4.015(8) s , β− (99.96%) , 147Ce , rowspan=2, (5/2+) , rowspan=2, , rowspan=2, , - , β−, n (.04%) , 146Ce , - , rowspan=2, 148La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 91 , rowspan=2, 147.93223(6) , rowspan=2, 1.26(8) s , β− (99.85%) , 148Ce , rowspan=2, (2−) , rowspan=2, , rowspan=2, , - , β−, n (.15%) , 147Ce , - , rowspan=2, 149La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 92 , rowspan=2, 148.93473(34)# , rowspan=2, 1.05(3) s , β− (98.6%) , 149Ce , rowspan=2, 5/2+# , rowspan=2, , rowspan=2, , - , β−, n (1.4%) , 148Ce , - , rowspan=2, 150La , rowspan=2 style="text-align:right" , 57 , rowspan=2 style="text-align:right" , 93 , rowspan=2, 149.93877(43)# , rowspan=2, 510(30) ms , β− (97.3%) , 150Ce , rowspan=2, (3+) , rowspan=2, , rowspan=2, , - , β−, n (2.7%) , 149Ce , - , 151La , style="text-align:right" , 57 , style="text-align:right" , 94 , 150.94172(43)# , 300# ms 300 ns, β− , 151Ce , 5/2+# , , , - , 152La , style="text-align:right" , 57 , style="text-align:right" , 95 , 151.94625(43)# , 200# ms 300 ns, β− , 152Ce , , , , - , 153La , style="text-align:right" , 57 , style="text-align:right" , 96 , 152.94962(64)# , 150# ms 300 ns, β− , 153Ce , 5/2+# , , , - , 154La , style="text-align:right" , 57 , style="text-align:right" , 97 , 153.95450(64)# , 100# ms , β− , 154Ce , , , , - , 155La , style="text-align:right" , 57 , style="text-align:right" , 98 , 154.95835(86)# , 60# ms , β− , 155Ce , 5/2+# , ,References
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lant ...