Juliá–Colonna epoxidation on:
[Wikipedia]
[Google]
[Amazon]
The Juliá–Colonna epoxidation is an asymmetric poly- In the original triphasic protocol, the
In the original triphasic protocol, the
 The
The

The original poly-leucine catalysts were formed by reacting leucine-N-carboxyanhydrides with an initiator such as an amine, an alcohol or water (Figure 5). In triphasic systems, the polymer catalyst must be soaked in the organic solvent and peroxide solution to generate a gel prior to reaction.Adger, B. M.; Barkley, J. V.; Bergeron, S.; Cappi, M. W.; Flowerdew, B. E.; Jackson, M. P.; McCague, R.; Nugent, T. C.; Roberts, S. M., "Improved procedure for Julia–Colonna asymmetric epoxidation of α,β-unsaturated ketones: total synthesis of diltiazem and Taxol (TM) side-chain". ''J. Chem. Soc.-Perkin Trans. 1'' 1997, (23), 3501–3507. – Especially in biphasic systems, reaction time may be reduced and enantioselectivity increased by activating the catalyst with NaOH prior to reaction. Furthermore, in biphasic systems the polymer may be immobilized on
leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
catalyzed nucleophilic epoxidation
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly reactive, more so than other ...
of electron deficient olefins in a triphasic system. The reaction was reported by Sebastian Juliá at the Chemical Institute of Sarriá in 1980, with further elaboration by both Juliá and Stefano Colonna (Istituto di Chimica Industriale dell'Università, Milan, Italy).
: In the original triphasic protocol, the
In the original triphasic protocol, the chalcone
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. They are widely known bioactive substances, fluorescent materi ...
substrate is soluble in the organic phase, generally toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
or carbon tetrachloride
Carbon tetrachloride, also known by many other names (such as carbon tet for short and tetrachloromethane, also IUPAC nomenclature of inorganic chemistry, recognised by the IUPAC), is a chemical compound with the chemical formula CCl4. It is a n ...
. The alkaline hydrogen peroxide oxidant is soluble primarily in the aqueous phase, and the reaction occurs at the insoluble polymer layer at the interface of the two phases. Alternative biphasic and monophasic protocols have been developed with increased substrate accessibility and reaction rate.
The efficient enantioselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
catalytic epoxidation under mild conditions is of great synthetic utility. Not only are epoxides
In organic chemistry, an epoxide is a cyclic ether, where the ether forms a three-atom Ring (chemistry), ring: two atoms of carbon and one atom of oxygen. This triangular structure has substantial ring strain, making epoxides highly Reactivity ( ...
effective synthons for a range of transformations, they have a significant presence in natural products structures. Furthermore, the reaction has been effectively scaled up to industrially useful levels, with work conducted notably by Bayer
Bayer AG (English: , commonly pronounced ; ) is a German multinational pharmaceutical and biotechnology company and is one of the largest pharmaceutical companies and biomedical companies in the world. Headquartered in Leverkusen, Bayer' ...
and Evonik
, products = Chemicals
, revenue = {{Decrease €15.2 billion (2024){{cite web , url= https://www.evonik.com/en/investor-relations/Reporting.html#tabs-b118b10b7a-item-2d4ab0c2ad-tab , title=Evonik Financial Report 2024 , publisher=Evonik Industri ...
. Finally, the enzyme-like activity of the poly-amino acid segments is suggestive of a role of the reaction in the prebiotic origin of life.
Reaction mechanism
The Juliá–Colonna epoxidation is an asymmetric nucleophilic epoxidation of electron-deficientolefins
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of P ...
such as α,β-unsaturated ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
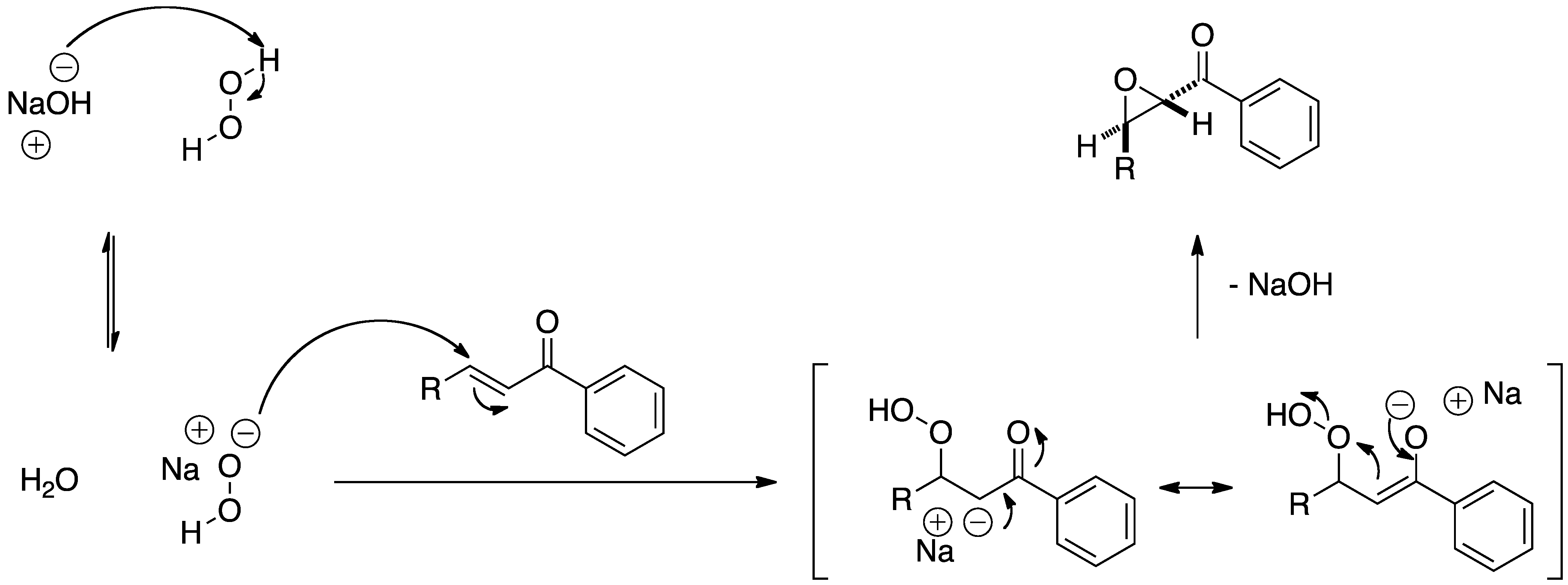
. The general mechanism shown in Figure 2 applies to all nucleophilic epoxidations but is controlled in this reaction by the poly-leucine catalyst.
 The
The hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds of the form ROOH, where R stands for any group, typically Organic compound, organic, which contain the hydroperoxy functional group (). Hydroperoxide also refers to the hydroperoxide anio ...
anion and chalcone assemble in a complex with the poly-leucine catalyst before reacting to form a peroxide enolate intermediate. The intermediate subsequently closes, as controlled by the catalyst structure, to form the epoxide product stereoselectively.
Ternary complex formation
The poly-leucine strands demonstrate enzyme-like kinetics with a first-order dependence on and eventual saturation with both the hydroperoxide anion (KM= 30 mM) and the olefin substrate (KM=110 mM.) Kinetic study suggests that the reaction proceeds by random steady-state formation of a ternary (polyleucine+hydroperoxide anion+olefin)complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
. Both substrates must bind prior to reaction, and while either may bind first, initial hydroperoxide binding is kinetically preferred. The rapid equilibrium enabling complex formation is followed by the rate-limiting formation of the peroxide enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the Organic synthesis, synthesis of organic compounds.
Bonding and structure
Enolate ...
(Figure 3).Carrea, G.; Colonna, S.; Meek, A. D.; Ottolina, G.; Roberts, S. M., "Kinetics of chalcone oxidation by peroxide anion catalysed by poly-L-leucine". ''Chem. Comm.'' 2004, (12), 1412-1413.
Mechanistic origin of stereoselectivity
All of the reactants associate with the polyleucine catalyst prior to reaction to form the hydroperoxide enolate intermediate. The catalyst orients the reactants and, even more significantly, the peroxide enolate intermediate by a series ofhydrogen bonding
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, Covalent bond, covalently b ...
interactions with the four N-terminal amino groups in the poly-leucine α-helix
An alpha helix (or α-helix) is a sequence of amino acids in a protein that are twisted into a coil (a helix).
The alpha helix is the most common structural arrangement in the Protein secondary structure, secondary structure of proteins. It is al ...
. While other models have been proposed,Berkessel, A.; Gasch, N.; Glaubitz, K.; Koch, C., "Highly enantioselective enone epoxidation catalyzed by short solid phase-bound peptides: Dominant role of peptide helicity". ''Org. Lett.'' 2001, ''3'' (24), 3839–3842. computations by Kelly et al. have suggested that the NH-2, NH-3, and NH-4 form an isosceles triangle available for hydrogen bonding as an intermediate-stabilizing oxyanion hole
An oxyanion hole is a pocket in the active site of an enzyme that stabilizes transition state negative charge on a deprotonation, deprotonated oxygen or alkoxide. The pocket typically consists of backbone amides or positively charged residues. Sta ...
. While olefin binding to either the ''endo'' or ''exo'' face of the helix is sterically allowed, only ''endo'' binding orients the NH-4 group to bind with the hydroperoxide moiety allowing for hydroxide displacement in the final reaction step (Figure 4).Kelly, D. R.; Roberts, S. M., The mechanism of polyleucine catalysed asymmetric epoxidation". ''Chem. Comm.'' 2004, (18), 2018-2020.
Catalyst
Poly-amino acid selection
Enantioselectivity is maximized by poly-amino acid sequences containing the greatest α-helical content; these include poly-leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
and poly-alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group sid ...
. Both poly-L- and poly-D-amino acids are available and cause the opposite stereoinduction.Bentley, P. A.; Cappi, M. W.; Flood, R. W.; Roberts, S. M.; Smith, J. A., Towards a mechanistic insight into the Julia-Colonna asymmetric epoxidation of α,β-unsaturated ketones using discrete lengths of poly-leucine. ''Tetrahedron Lett.'' 1998, ''39'' (50), 9297–9300.
Catalyst generation

The original poly-leucine catalysts were formed by reacting leucine-N-carboxyanhydrides with an initiator such as an amine, an alcohol or water (Figure 5). In triphasic systems, the polymer catalyst must be soaked in the organic solvent and peroxide solution to generate a gel prior to reaction.Adger, B. M.; Barkley, J. V.; Bergeron, S.; Cappi, M. W.; Flowerdew, B. E.; Jackson, M. P.; McCague, R.; Nugent, T. C.; Roberts, S. M., "Improved procedure for Julia–Colonna asymmetric epoxidation of α,β-unsaturated ketones: total synthesis of diltiazem and Taxol (TM) side-chain". ''J. Chem. Soc.-Perkin Trans. 1'' 1997, (23), 3501–3507. – Especially in biphasic systems, reaction time may be reduced and enantioselectivity increased by activating the catalyst with NaOH prior to reaction. Furthermore, in biphasic systems the polymer may be immobilized on
polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It i ...
, polyethylene glycol
Polyethylene glycol (PEG; ) is a polyether compound derived from petroleum with many applications, from industrial manufacturing to medicine. PEG is also known as polyethylene oxide (PEO) or polyoxyethylene (POE), depending on its molecular wei ...
(PEG), or silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
gel and formed into a paste.
Catalyst secondary structure
The active component of the catalyst assumes an α-helical structure where the four to five N-terminal residues are actively involved in catalysis. While active catalysts have been generated from scalemic leucine, consistentenantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
ic content must be maintained through the N-terminal region to give appropriate handedness to the structure. While the greatest enantioselectivity was originally observed when n=30 residues, a 10-mer leucine polypeptide is of sufficient length to provide significant enantioselectivity Following improvement of the original procedure, greater enantioselectivity has been observed for lower molecular weight polymers, presumably due to the greater number of N-termini available per mass used.
Scope
The Juliá–Colonna epoxidation of electron-deficient olefins was originally demonstrated with chalcones, but it was soon extended to other systems with electron withdrawing moieties such as α,β-unsaturated ketones, esters, and amides. The reaction has also demonstrated efficiency withsulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
substrates, and the scope of the reaction is being expanded with further methodological investigation.Lopez-Pedrosa, J. M.; Pitts, M. R.; Roberts, S. M.; Saminathan, S.; Whittall, J., "Asymmetric epoxidation of some arylalkenyl sulfones using a modified Julia–Colonna procedure". ''Tetrahedron Lett.'' 2004, ''45'' (26), 5073–5075.
Several classes of substrates, however, are not suitable for the Juliá–Colonna Epoxidation. These include:
* compounds sensitive to hydroxide.
* compounds with acidic protons on the α or α’ positions.
* electron rich olefins.
The nucleophilic epoxidation is naturally complementary in scope to electrophilic epoxidations such as the Sharpless epoxidation and Jacobsen epoxidation.
Stereoselectivity
Catalyst structure
The stereoinduction of the Juliá–Colonna epoxidation is dependent on the α-helical secondary structure of the poly-leucine catalyst. While the consistent stereochemistry of the N-terminal amino acids is necessary for this induction, even a 10-mer leucine polypeptide is of sufficient length to provide significant enantioselectivity.Chiral amplification by scalemic catalysts
This dependence solely on the N-terminal region of the helix is most pronounced in enantioselective stereoinduction by scalemic catalysts. Even a 40% enantiomeric excess of L vs. D-leucine in catalyst formation can yield the same enantiomeric enriched epoxide as the enantiopure catalyst. The relationship between catalyst and product enantiopurity can be closely approximated with a Bernoullian statistical model: een=(Ln-Dn)/(Ln+Dn) where L and D are the proportions of L- and D-leucine used to generate the catalytic polymers and n is the length of the catalytic component. Chiral amino acids, including leucine, have been generated in electrical discharge experiments designed to mimic the prebiotic conditions on Earth, and they have been found in scalemic mixtures in meteorites. It has been suggested that poly-amino acid fragments analogous to the Juliá–Colonna catalyst may have been initiated byimidazole
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole.
Many natural products, ...
or cyanide
In chemistry, cyanide () is an inorganic chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.
Ionic cyanides contain the cyanide anion . This a ...
derivatives, and the resulting fragments may have played a catalytic role in the origin of enantiomeric enrichment ubiquitous in life today.
Variations
Silica-grafted catalysts
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
-grafted polyleucine has been shown to effectively catalyze epoxidation of α,β-unsaturated aromatic ketones. The silica graft allows for the catalyst to be easily recovered with only mild loss of activity and is particularly useful for scale-up reactions.Yi, H.; Zou, G.; Li, Q.; Chen, Q.; Tang, J.; He, M. Y., "Asymmetric epoxidation of alpha,beta-unsaturated ketones catalyzed by silica-grafted poly-(L)-leucine catalysts". ''Tetrahedron Lett.'' 2005, ''46'' (34), 5665–5668.
Biphasic (non-aqueous) reaction conditions
For the alternative biphasic protocol, the olefin substrate is dissolved intetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF) along with the urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest am ...
hydrogen peroxide
In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...
(UHP) oxidant and a tertiary amine base such as 8-diazabicyclo .4.0ndec-7-ene (DBU.) The immobilized polymer catalyst forms a paste which serves as the reaction site. The two phase reaction conditions extended the range of enones to which the reaction could be applied.
Monophasic reaction conditions with PEG-immobilized polyleucine
A soluble initiator O,O′-bis(2-aminoethyl)polyethylene glycol (diaminoPEG) for poly-leucine assembly was utilized to generate a THF-soluble triblockpolymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
. Utilization of this catalyst in homogeneous reaction conditions enabled marked extension of the methodology to α,β-unsaturated ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
, diene
In organic chemistry, a diene ( ); also diolefin, ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nome ...
s, and ''bis''-dienes.
Phase transfer co-catalysis
Addition of tetrabutylammonium bromide as aphase transfer catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homog ...
dramatically increases the rate of reaction. The co-catalyst is presumed to increase the concentration of the peroxide oxidant in the organic phase enabling more efficient access to the reactive ternary complex.Geller, T.; Gerlach, A.; Kruger, C. M.; Militzer, H. C., "Novel conditions for the Julia–Colonna epoxidation reaction providing efficient access to chiral, nonracemic epoxides". ''Tetrahedron Lett.'' 2004, ''45'' (26), 5065–5067. These conditions were developed for application to two phase systems but also function for three phase systems and have been utilized up to the 100g scale
Scale-up
Immobilized catalysts have been used inmembrane reactor
A membrane reactor is a physical device that combines a chemical conversion process with a membrane separation process to add reactants or remove products of the reaction.
Chemical reactors making use of membranes are usually referred to as mem ...
s and are being investigated for application to continuous flow fixed bed reactors.
Applications to synthesis
Total synthesis of Diltiazem
Adger et al. utilized the biphasic Juliá–Colonna epoxidation with immobilized poly-L-leucine (I-PLL) and urea hydrogen peroxide (UHP), and 8-diazabicyclo .4.0ndec-7-ene (DBU) as the key step in the efficient synthesis ofDiltiazem
Diltiazem, sold under the brand name Cardizem among others, is a nondihydropyridine calcium channel blocker medication used to treat high blood pressure, angina, and certain heart arrhythmias. It may also be used in hyperthyroidism if beta b ...
(Figure 6.) Diltiazem is a commercially available pharmaceutical which acts as a calcium channel
A calcium channel is an ion channel which shows selective permeability to calcium ions. It is sometimes synonymous with voltage-gated calcium channel, which are a type of calcium channel regulated by changes in membrane potential. Some calcium chan ...
blocker.Total synthesis of (+)-clausenamide
Cappi et al. utilized the Juliá–Colonna epoxidation with PEG-immobilized poly-L-leucine (PEG-PLL) and DABCO hydrogen peroxide (DABCO-H2O2) or urea hydrogen peroxide (UHP) in a miniature fixed-bed continuous flow reactor system (Figure 7.) This protocol was exploited to synthesize (+)-clausenamide as a proof of concept in the development of the novel reaction protocol; (+)-clausenamide exhibits anti-amnesiac and hepatoprotective activity.Cappi, M. W.; Chen, W. P.; Flood, R. W.; Liao, Y. W.; Roberts, S. M.; Skidmore, J.; Smith, J. A.; Williamson, N. M., "New procedures for the –Colonna asymmetric epoxidation: synthesis of (+)-clausenamide". ''Chem. Comm.'' 1998, (10), 1159-1160.Total synthesis of (+)-goniotriol 7, (+)-goniofufurone 8, (+)-8-acetylgoniotriol 9 and gonio-pypyrone
Chen et al. utilized the biphasic Juliá–Colonna Epoxidation protocol with urea hydrogen peroxide (UHP), poly-L-leucine (PLL), and 8-diazabicyclo .4.0ndec-7-ene (DBU) as a key step in the synthesis of a family of styryl lactones isolated from '' Goniothalamus giganteus'' (Figure 8.) These compounds, including (+)-goniotriol 7, (+)-goniofufurone 8, (+)-8-acetylgoniotriol 9 and gonio-pypyrone, have demonstratedcytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are toxic metals, toxic chemicals, microbe neurotoxins, radiation particles and even specific neurotransmitters when the system is out of balance. Also some types of dr ...
activity against human tumor cells.Chen, W. P.; Roberts, S. M., "Julia–Colonna asymmetric epoxidation of furyl styryl ketone as a route to intermediates to naturally-occurring styryl lactones". ''J. Chem. Soc.-Perkin Trans. 1'' 1999, (2), 103–105. See also
*Prilezhaev reaction
The Prilezhaev reaction, also known as the Prileschajew reaction or Prilezhaev epoxidation, is the chemical reaction of an alkene with a peroxy acid to form epoxides. It is named after Nikolai Prilezhaev, who first reported this reaction in 1909 ...
* Jørgensen epoxidation
* Asymmetric nucleophilic epoxidation
References
External links
* https://www.organic-chemistry.org/Highlights/2004/22November.shtm {{DEFAULTSORT:Julia-Colonna epoxidation Epoxidation reactions Oxygen heterocycle forming reactions Name reactions