|
Juliá–Colonna Epoxidation
The Juliá–Colonna epoxidation is an asymmetric poly-leucine catalyzed nucleophilic epoxidation of electron deficient olefins in a triphasic system. The reaction was reported by Sebastian Juliá at the Chemical Institute of Sarriá in 1980, with further elaboration by both Juliá and Stefano Colonna (Istituto di Chimica Industriale dell'Università, Milan, Italy). : In the original triphasic protocol, the chalcone substrate is soluble in the organic phase, generally toluene or carbon tetrachloride. The alkaline hydrogen peroxide oxidant is soluble primarily in the aqueous phase, and the reaction occurs at the insoluble polymer layer at the interface of the two phases. Alternative biphasic and monophasic protocols have been developed with increased substrate accessibility and reaction rate. The efficient enantioselective catalytic epoxidation under mild conditions is of great synthetic utility. Not only are epoxides effective synthons for a range of transformations, they hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Like valine and isoleucine, leucine is a branched-chain amino acid. The primary metabolic end products of leucine metabolism are acetyl-CoA and acetoacetate; consequently, it is one of the two exclusively ketogenic amino acids, with lysine being the other. It is the most import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Nucleophilic Epoxidation
Nucleophilic epoxidation is the formation of epoxides from electron-deficient double bonds through the action of nucleophilic oxidants. Nucleophilic epoxidation methods represent a viable alternative to electrophilic methods, many of which do not epoxidize electron-poor double bonds efficiently. Although the most commonly used asymmetric epoxidation methods (the Sharpless-Katsuki, and Jacobsen epoxidations) rely on the catalytic reactivity of electrophilic oxidants, nucleophilic oxygen sources substituted with a suitable leaving group can also act as epoxidation reagents. The classic example, the Weitz-Scheffer reaction employs hydrogen peroxide under basic conditions (Z = OH below). Other notable examples have employed hypochlorites (Z = Cl) and chiral peroxides (Z = OR*). Asymmetric versions of the above reaction have taken advantage of a number of strategies for achieving asymmetric induction. The highest yielding and most enantioselective methods include: * Use of stoichiometr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Multiphasic Liquid
A multiphasic liquid is a mixture consisting of more than two immiscible liquid phases. Biphasic mixtures consisting of two immiscible phases are very common and usually consist of an organic solvent and an aqueous phase ("oil and water"). Multiphasic liquids can be used for selective liquid-liquid extractions or for decorative purposes, e.g. in cosmetics. While it is possible to get multilayered phases by layering nonpolar and aqueous phases of decreasing densities on top of each other, these phases will not separate after mixing like true multiphasic liquids. Compositions The following types of multiphasic liquids exist: Triphasic systems * Nonpolar solvent / aqueous biphasic mixture *: ''e.g. using hexane, heptane, cyclohexane, or mineral oil as the nonpolar solvent'' ** Nonpolar solvent / polar solvent / salt / water **: ''e.g. 100 ml mineral oil, 100 ml isopropanol, 75 ml water, 35 g calcium chloride'' ** Nonpolar solvent / water-soluble polymer A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
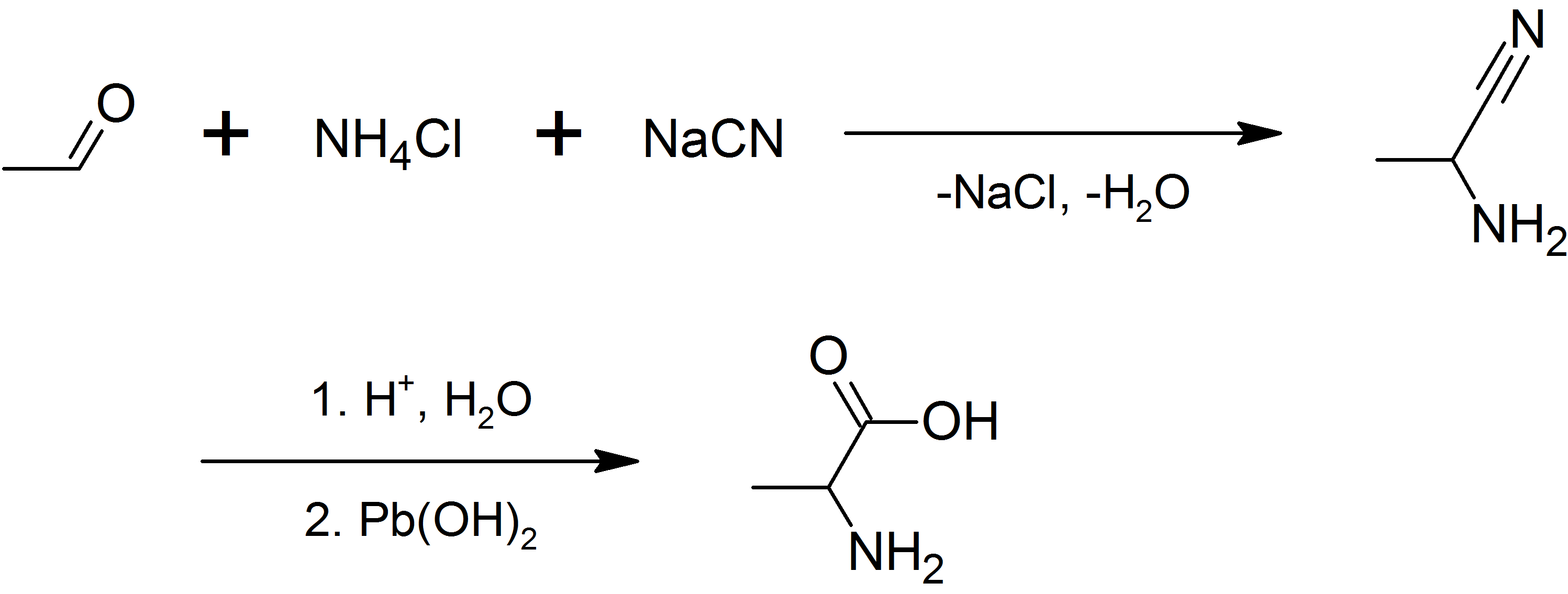
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxyanion Hole
An oxyanion hole is a pocket in the active site of an enzyme that stabilizes transition state negative charge on a deprotonated oxygen or alkoxide. The pocket typically consists of backbone amides or positively charged residues. Stabilising the transition state lowers the activation energy necessary for the reaction, and so promotes catalysis. For example, proteases such as chymotrypsin contain an oxyanion hole to stabilise the tetrahedral intermediate anion formed during proteolysis and protects substrate's negatively charged oxygen from water molecules. Additionally, it may allow for insertion or positioning of a substrate, which would suffer from steric hindrance Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ... if it could not occupy the hole (such as 2,3-Bisphosphoglycerate, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Helix
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues earlier along the protein sequence. The alpha helix is also called a classic Pauling–Corey–Branson α-helix. The name 3.613-helix is also used for this type of helix, denoting the average number of residues per helical turn, with 13 atoms being involved in the ring formed by the hydrogen bond. Among types of local structure in proteins, the α-helix is the most extreme and the most predictable from sequence, as well as the most prevalent. Discovery In the early 1930s, William Astbury showed that there were drastic changes in the X-ray fiber diffraction of moist wool or hair fibers upon significant stretching. The data suggested that the unstretched fibers had a coiled molecular structure with a characteristic repeat of ≈. Astb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enolate
In organic chemistry, enolates are organic anions derived from the deprotonation of carbonyl () compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds. Bonding and structure Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both an alkoxide and a carbanion. Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates. Preparation Deprotonation of enolizable ketones, aromatic alcohols, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA). Often, as in conventional Claisen condensations, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide bases. Under such conditions, they exist in low concent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ternary Complex
A ternary complex is a protein complex containing three different molecules that are bound together. In structural biology, ''ternary complex'' can also be used to describe a crystal containing a protein with two small molecules bound, for example cofactor and substrate; or a complex formed between two proteins and a single substrate. In Immunology, ''ternary complex'' can refer to the MHC–peptide–T-cell-receptor complex formed when T cells recognize epitopes of an antigen. Some other example can be taken like ternary complex while eukaryotic translation, in which ternary complex is composed of eIF-3 & eIF-2 + Ribosome 40s subunit+ tRNAi. A ternary complex can be a complex formed between two substrate molecules and an enzyme. This is seen in multi-substrate enzyme-catalyzed reactions where two substrates and two products can be formed. The ternary complex is an intermediate between the product formation in this type of enzyme-catalyzed reactions. An example for a ternary compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Kinetics
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying the conditions of the reaction are investigated. Studying an enzyme's kinetics in this way can reveal the catalytic mechanism of this enzyme, its role in metabolism, how its activity is controlled, and how a drug or a modifier ( inhibitor or activator) might affect the rate. An enzyme (E) is typically a protein molecule that promotes a reaction of another molecule, its substrate (S). This binds to the active site of the enzyme to produce an enzyme-substrate complex ES, and is transformed into an enzyme-product complex EP and from there to product P, via a transition state ES*. The series of steps is known as the mechanism: : E + S ⇄ ES ⇄ ES* ⇄ EP ⇄ E + P This example assumes the simplest case of a reaction with one substrate and one product. Such cases exist: for example, a mutase such as phosphoglucomutase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroperoxide
Hydroperoxides or peroxols are Chemical compound, compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. Organic hydroperoxides can either intentionally or unintentionally initiate explosive polymerisation in materials with Unsaturated bond, unsaturated chemical bonds. Properties The O−O bond length in peroxides is about 1.45 Ångström, Å, and the R−O−O angles (R = H, C) are about 110° (water-like). Characteristically, the C−O−O−H dihedral angles are about 120°. The O−O bond is relatively weak, with a bond dissociation energy of , less than half the strengths of C−C, C−H, and C−O bonds. Hydroperoxides are typically more volatile than the corresponding alcohols: * tert-Butyl hydroperoxide, ''tert''-BuOOH (b.p. 36°C) vs tert-Butyl alcohol, ''tert''-BuOH (b.p. 82-83°C) * Methyl hydroperoxide, CH3O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |