Isobutylparaben on:
[Wikipedia]
[Google]
[Amazon]
Parabens are a class of widely used
Antiperspirants and Breast Cancer Risk A 2005 review concluded "it is biologically implausible that parabens could increase the risk of any estrogen-mediated endpoint, including effects on the male reproductive tract or
 In one New York
In one New York

 In addition to parent parabens, paraben degradation products that form throughout WWTP stages present a concern to the environment, including mono- and di- chlorinated parabens. When paraben-containing products are washed down the drain, parabens have the potential to undergo chlorination reactions.Mao Q., Ji F., Wang W., Wang Q., Hu Z., Yuan S. (2016) Chlorination of parabens: reaction kinetics and transformation product identification. Environ. Sci. Polut. Res. 23, 23081–23091. This reaction can occur with free chlorine present in tap water or with
In addition to parent parabens, paraben degradation products that form throughout WWTP stages present a concern to the environment, including mono- and di- chlorinated parabens. When paraben-containing products are washed down the drain, parabens have the potential to undergo chlorination reactions.Mao Q., Ji F., Wang W., Wang Q., Hu Z., Yuan S. (2016) Chlorination of parabens: reaction kinetics and transformation product identification. Environ. Sci. Polut. Res. 23, 23081–23091. This reaction can occur with free chlorine present in tap water or with

 Another significant paraben degradation product is
Another significant paraben degradation product is
 Paraben stability in sewage sludge is relatively high due to their ability to bind with organic matter. Soil adsorption coefficient values were calculated by the U.S. Environmental Protection Agency as 1.94 (methylparaben), 2.20 (ethylparaben), 2.46 (propylparaben), and 2.72 (butylparaben), all of which suggest that parabens have the ability to adhere to the organic portion of sediment and sludge, and thus, persist environmentally.
Chlorinated parabens are removed from WWTPs with only 40% efficiency in comparison to 92–98% efficiency of parent parabens. The decrease in removal efficiency can be attributed to the decreased
Paraben stability in sewage sludge is relatively high due to their ability to bind with organic matter. Soil adsorption coefficient values were calculated by the U.S. Environmental Protection Agency as 1.94 (methylparaben), 2.20 (ethylparaben), 2.46 (propylparaben), and 2.72 (butylparaben), all of which suggest that parabens have the ability to adhere to the organic portion of sediment and sludge, and thus, persist environmentally.
Chlorinated parabens are removed from WWTPs with only 40% efficiency in comparison to 92–98% efficiency of parent parabens. The decrease in removal efficiency can be attributed to the decreased
 Ozonation is an advanced treatment technique that has been considered as a possible method to limit the amount of parabens, chlorinated parabens, and PHBA that are accumulating in the environment. Ozone is an extremely powerful oxidant that oxidizes parabens and makes them easier to remove once subsequently passed through a filter.Tay K. S., Rahman N. A., Abas M. R. B. (2010) Ozonation of parabens in aqueous solutions: kinetics and mechanism of degradation. Chemosphere. 81, 1446–1453. Due to the electrophilic nature of ozone, it can easily react with the aromatic paraben ring to form hydroxylated products. Ozonation is generally regarded as a less dangerous method of disinfection than chlorination, though ozonation requires more cost considerations. Ozonation has demonstrated great efficacy in the removal of parabens (98.8–100%) and a slightly lower efficacy of 92.4% for PHBA. A moderately lower rate of removal, however, is observed for chlorinated parabens (59.2–82.8%). A proposed reaction mechanism for the removal of parabens by ozonation is detailed mechanistically.
Ozonation is an advanced treatment technique that has been considered as a possible method to limit the amount of parabens, chlorinated parabens, and PHBA that are accumulating in the environment. Ozone is an extremely powerful oxidant that oxidizes parabens and makes them easier to remove once subsequently passed through a filter.Tay K. S., Rahman N. A., Abas M. R. B. (2010) Ozonation of parabens in aqueous solutions: kinetics and mechanism of degradation. Chemosphere. 81, 1446–1453. Due to the electrophilic nature of ozone, it can easily react with the aromatic paraben ring to form hydroxylated products. Ozonation is generally regarded as a less dangerous method of disinfection than chlorination, though ozonation requires more cost considerations. Ozonation has demonstrated great efficacy in the removal of parabens (98.8–100%) and a slightly lower efficacy of 92.4% for PHBA. A moderately lower rate of removal, however, is observed for chlorinated parabens (59.2–82.8%). A proposed reaction mechanism for the removal of parabens by ozonation is detailed mechanistically.
preservatives
A preservative is a substance or a chemical that is added to products such as food products, beverages, pharmaceutical drugs, paints, biological samples, cosmetics, wood, and many other products to prevent decomposition by microbial growth or b ...
in cosmetic
Cosmetic may refer to:
* Cosmetics, or make-up, substances to enhance the beauty of the human body, apart from simple cleaning
*Cosmetic, an adjective describing beauty, aesthetics, or appearance, especially concerning the human body
*Cosmetic, ...
and pharmaceutical
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy ( pharmacotherapy) is an important part of the medical field and ...
products. Chemically, they are a series of parahydroxybenzoates or ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s of parahydroxybenzoic acid (also known as 4-hydroxybenzoic acid
4-Hydroxybenzoic acid, also known as ''p''-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar ...
). Parabens are effective preservatives in many types of formulas. These compounds, and their salts, are used primarily for their bactericidal
A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance which kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics.
However, material surfaces can also have bactericidal properties based solely on their ...
and fungicidal
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality ...
properties. They are found in shampoo
Shampoo () is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in solid bar format. Shampoo is used by applying it to wet hair, massaging the product into th ...
s, commercial moisturizer
A moisturizer, or emollient, is a cosmetic preparation used for protecting, moisturizing, and lubricating the skin. These functions are normally performed by sebum produced by healthy skin. The word "emollient" is derived from the Latin verb ''m ...
s, shaving gel
Shaving cream or shave cream is a category of cream cosmetics used for shaving preparation. The purpose of shaving cream is to soften the hair by providing lubrication.
Different types of shaving creams include Aerosol spray, aerosol shaving ...
s, personal lubricant
Personal lubricants (colloquially termed lube) are specialized lubricants used during sexual acts, such as intercourse and masturbation, to reduce friction to or between the penis and vagina, anus or other body parts or applied to sex toys to re ...
s, topical
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of class ...
/parenteral
A route of administration in pharmacology and toxicology is the way by which a drug, fluid, poison, or other substance is taken into the body.
Routes of administration are generally classified by the location at which the substance is applied. ...
pharmaceuticals, suntan products, makeup
The asterisk ( ), from Late Latin , from Ancient Greek , ''asteriskos'', "little star", is a typographical symbol. It is so called because it resembles a conventional image of a heraldic star.
Computer scientists and mathematicians often vo ...
, and toothpaste
Toothpaste is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth ...
. They are also used as food preservatives
Food preservation includes processes that make food more resistant to microorganism growth and slow the oxidation of fats. This slows down the decomposition and rancidification process. Food preservation may also include processes that inhibit ...
.
Certain parabens have been linked to cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal bl ...
.
Mode of action
Parabens are active against a broad spectrum ofmicroorganisms
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
. However, their antibacterial
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention ...
mode of action is not well understood. They are thought to act by disrupting membrane transport processes or by inhibiting synthesis of DNA and RNA or of some key enzymes, such as ATPases and phosphotransferases, in some bacterial species. Propylparaben is considered more active against more bacteria than methylparaben.
The stronger antibacterial action of propylparaben may be due to its greater solubility in the bacterial membrane, which may allow it to reach cytoplasmic targets in greater concentrations. However, since a majority of the studies on the mechanism of action of parabens suggest that their antibacterial action is linked to the membrane, it is possible that its greater lipid solubility disrupts the lipid bilayer, thereby interfering with bacterial membrane transport processes and perhaps causing the leakage of intracellular constituents.
Chemistry
Parabens areester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
s of ''para''-hydroxy''ben''zoic acid, from which the name is derived. Common parabens include methylparaben
Methylparaben, also methyl paraben, one of the parabens, is a preservative with the chemical formula CH3(C6H4(OH)COO). It is the methyl ester of ''p''-hydroxybenzoic acid.
Natural occurrences
Methylparaben serves as a pheromone for a variety ...
(E number
E numbers ("E" stands for "Europe") are codes for substances used as food additives, including those found naturally in many foods such as vitamin C, for use within the European Union (EU) and European Free Trade Association (EFTA). Commonly ...
E218), ethylparaben
Ethylparaben (ethyl ''para''-hydroxybenzoate) is the ethyl ester of ''p''-hydroxybenzoic acid. Its formula is HO-C6H4-CO-O-CH2CH3. It is a member of the class of compounds known as parabens.
It is used as an antifungal preservative. As a food ...
(E214), propylparaben
Propylparaben, the ''n''-propyl ester of ''p''-hydroxybenzoic acid, occurs as a natural substance found in many plants and some insects, although it is manufactured synthetically for use in cosmetics, pharmaceuticals, and foods. It is a member ...
(E216), butylparaben
Butylparaben, or butyl ''p''-hydroxybenzoate, is an organic compound with the formula . It is a white solid that is soluble in organic solvents. It has proven to be a highly successful antimicrobial preservative in cosmetics. It is also used in ...
and heptylparaben
Heptylparaben (heptyl ''p''-hydroxybenzoate) is a compound with formula C7H15(C6H4OHCOO). It is a paraben which is the heptyl ester of ''p''-hydroxybenzoic acid.
Heptylparaben has also been found to be produced in some microorganisms including ...
(E209). Less common parabens include isobutylparaben, isopropylparaben
Isopropylparaben is a paraben.
Synthesis
Isopropylparaben has been prepared via the stepwise addition of isopropanol, thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colour ...
, benzylparaben and their sodium salts. The general chemical structure of a paraben is shown at the top right of this page, where R symbolizes an alkyl group such as methyl, ethyl, propyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often ...
or butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, giv ...
.
Synthesis
All commercially used parabens are synthetically produced, although some are identical to those found in nature. They are produced by theesterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glyceride ...
of ''para''-hydroxybenzoic acid with the appropriate alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
, such as methanol, ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a h ...
, or n-propanol
Propan-1-ol (also propanol, n-propyl alcohol) is a primary alcohol with the formula and sometimes represented as PrOH or ''n''-PrOH. It is a colorless liquid and an isomer of 2-propanol. It is formed naturally in small amounts during many ferme ...
. ''para''-Hydroxybenzoic acid is in turn produced industrially from a modification of the Kolbe-Schmitt reaction, using potassium phenoxide
Phenolates (also called phenoxides) are anions, salts, and esters of phenols. They may be formed by reaction of phenols with strong base.
Properties
Alkali metal phenolates, such as sodium phenolate hydrolyze in aqueous solution to form basic s ...
and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
.
Health considerations
Most of the available paraben toxicity data are from single-exposure studies, meaning one type of paraben in one type of product. According to paraben research this is relatively safe, posing only a negligible risk to the endocrine system. However, since many types of parabens in many types of products are used commonly, further assessment of the additive and cumulative risk of multiple paraben exposure from daily use of multiple cosmetic and/or personal care products is needed.FDA
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food s ...
states that they have no information that use of parabens in cosmetics has any effect on health. They continue to consider certain questions and evaluate data about parabens' possible health effects.
Allergic reactions
Parabens are, for the most part, non-irritating and non-sensitizing. Among people withcontact dermatitis
Contact dermatitis is a type of acute or chronic inflammation of the skin caused by exposure to chemical or physical agents. Symptoms of contact dermatitis can include itchy or dry skin, a red rash, bumps, blisters, or swelling. These rashes ar ...
or eczema
Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved can v ...
, less than 3% of patients were found to have a sensitivity to parabens. At least one case has been reported of an allergic reaction
Allergies, also known as allergic diseases, refer a number of conditions caused by the hypersensitivity of the immune system to typically harmless substances in the environment. These diseases include hay fever, food allergies, atopic derm ...
to parabens.
Breast cancer
TheAmerican Cancer Society
The American Cancer Society (ACS) is a nationwide voluntary health organization dedicated to eliminating cancer. Established in 1913, the society is organized into six geographical regions of both medical and lay volunteers operating in more tha ...
mentioned a 2004 study that found parabens in the breast tissue of mastectomy
Mastectomy is the medical term for the surgical removal of one or both breasts, partially or completely. A mastectomy is usually carried out to treat breast cancer. In some cases, women believed to be at high risk of breast cancer have the operat ...
patients but did not find parabens to be a cause of the cancers. Michael Thun of ACS stated that the effects of parabens would be minuscule compared to other risks "such as taking hormones after menopause
Menopause, also known as the climacteric, is the time in women's lives when menstrual periods stop permanently, and they are no longer able to bear children. Menopause usually occurs between the age of 47 and 54. Medical professionals often d ...
and being overweight".The American Cancer SocietyAntiperspirants and Breast Cancer Risk A 2005 review concluded "it is biologically implausible that parabens could increase the risk of any estrogen-mediated endpoint, including effects on the male reproductive tract or
breast cancer
Breast cancer is cancer that develops from breast tissue. Signs of breast cancer may include a lump in the breast, a change in breast shape, dimpling of the skin, milk rejection, fluid coming from the nipple, a newly inverted nipple, or ...
" and that "worst-case daily exposure to parabens would present substantially less risk relative to exposure to naturally occurring endocrine active chemicals in the diet such as the phytoestrogen
A phytoestrogen is a plant-derived xenoestrogen (see estrogen) not generated within the endocrine system, but consumed by eating plants or manufactured foods. Also called a "dietary estrogen", it is a diverse group of naturally occurring nonster ...
daidzein
Daidzein (7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one) is a naturally occurring compound found exclusively in soybeans and other legumes and structurally belongs to a class of compounds known as isoflavones. Daidzein and other isoflavones a ...
."
Estrogenic activity
Animal experiments have shown that parabens have weakestrogen
Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal a ...
ic activity, acting as xenoestrogen
Xenoestrogens are a type of xenohormone that imitates estrogen. They can be either synthetic or natural chemical compounds. Synthetic xenoestrogens include some widely used industrial compounds, such as PCBs, BPA, and phthalates, which have estro ...
s. In an ''in vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and ...
'' study, the effect of butylparaben was determined to be about 1/100,000th that of estradiol
Estradiol (E2), also spelled oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is responsible for the development o ...
, and was only observed at a dose level around 25,000 times higher than the level typically used to preserve products. The study also found that the ''in vivo'' estrogenic activity of parabens is reduced by about three orders of magnitude compared to ''in vitro
''In vitro'' (meaning in glass, or ''in the glass'') studies are performed with microorganisms, cells, or biological molecules outside their normal biological context. Colloquially called "test-tube experiments", these studies in biology and ...
'' activity.
The estrogenic activity of parabens increases with the length of the alkyl group. It is believed that propylparaben is estrogenic to a certain degree as well, though this is expected to be less than butylparaben by virtue of its less lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves li ...
nature. Since it can be concluded that the estrogenic activity of butylparaben is negligible under normal use, the same should be concluded for shorter analogs due to estrogenic activity of parabens increasing with the length of the alkyl group.
Regulation
TheEuropean Scientific Committee on Consumer Safety
European, or Europeans, or Europeneans, may refer to:
In general
* ''European'', an adjective referring to something of, from, or related to Europe
** Ethnic groups in Europe
** Demographics of Europe
** European cuisine
European cuisine co ...
(SCCS) reiterated in 2013 that methylparaben and ethylparaben are safe at the maximum authorized concentrations (up to 0.4% for one ester or 0.8% when used in combination). The SCCS concluded that the use of butylparaben and propylparaben as preservatives in finished cosmetic products is safe to the consumer, as long as the sum of their individual concentrations does not exceed 0.19%. Isopropylparaben, isobutylparaben, phenylparaben, benzylparaben and pentylparaben were banned by European Commission Regulation
A regulation is a legal act of the European Union that becomes immediately enforceable as law in all member states simultaneously. Regulations can be distinguished from directives which, at least in principle, need to be transposed into natio ...
(EU) No 358/2014.
Controversy
Concerns aboutendocrine disruptor
Endocrine disruptors, sometimes also referred to as hormonally active agents, endocrine disrupting chemicals, or endocrine disrupting compounds are chemicals that can interfere with endocrine (or hormonal) systems. These disruptions can cause ...
s have led consumers and companies to search for paraben-free alternatives. A common alternative has been phenoxyethanol
Phenoxyethanol is the organic compound with the formula C6H5OC2H4OH. It is a colorless oily liquid. It can be classified as a glycol ether and a phenol ether. It is a common preservative in vaccine formulations.
Use
Phenoxyethanol has germic ...
, but this has its own risks and has led to an FDA warning on inclusion in nipple creams.
Environmental considerations
Release into the environment
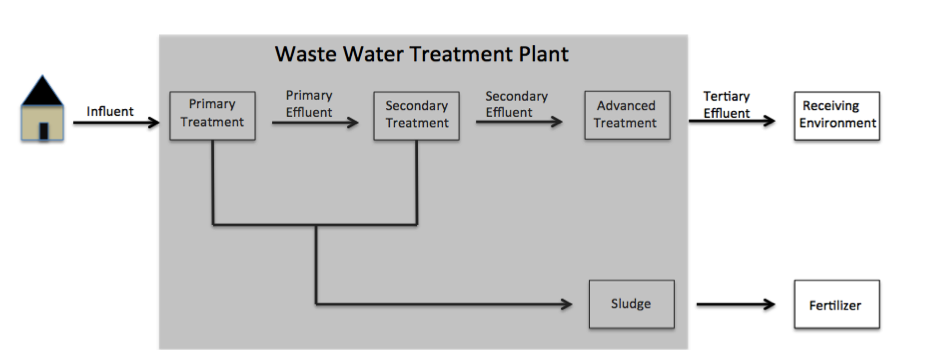
Paraben discharge into the environment is common due to its ubiquitous use in cosmetic products. A 2010 study on consumer available personal care products revealed that 44% of the tested products contain parabens. When washing these products off the human body, they flow down the drain and into community wastewater. Once this occurs, the potential for parabens to accumulate within aqueous and solid mediums materializes. Some of the most common paraben derivatives found in the environment include methylparaben, ethylparaben, propylparaben, and butylparaben.Li W., Shi Y., Gao L., Liu J., Cai Y. (2015). Occurrence, fate and risk assessment of parabens and their chlorinated derivatives in an advanced wastewater treatment plant. Journal of Hazardous Materials 300: 29–38. Parabens flow inwastewater
Wastewater is water generated after the use of freshwater, raw water, drinking water or saline water in a variety of deliberate applications or processes. Another definition of wastewater is "Used water from any combination of domestic, industri ...
to wastewater treatment plants (WWTP) as influent, where they are either removed, chemically altered, or released into the environment through sludge
Sludge is a semi-solid slurry that can be produced from a range of industrial processes, from water treatment, wastewater treatment or on-site sanitation systems. For example, it can be produced as a settled suspension obtained from conventional ...
or tertiary effluent.
 In one New York
In one New York wastewater treatment plant
Wastewater treatment is a process used to remove contaminants from wastewater and convert it into an effluent that can be returned to the water cycle. Once returned to the water cycle, the effluent creates an acceptable impact on the environmen ...
(WWTP), mass load of all parent paraben derivatives (methylparaben, ethylparaben, propylparaben, butylparaben, etc.) from influent wastewater was found to be 176 mg/day/1000 people.Wang W., Kannan K. (2016). Fate of Parabens and their Metabolites in two wastewater treatment plants in New York, United States. Environmental science & technology. 50: 1174–1181 When this value is used to estimate the amount of parabens entering WWTPs from the 8.5 million people currently residing in New York City for an entire year, a value of approximately 546 kg of parabens is calculated. Therefore, levels of paraben accumulation prove significant upon long-term observance. WWTPs eliminate between 92–98% of paraben derivatives; however, much of this removal is due to the formation of degradation products. Despite their reputed high elimination through WWTPs, various studies have measured high levels of paraben derivatives and degradation products persisting in the environment.
Formation of degradation products
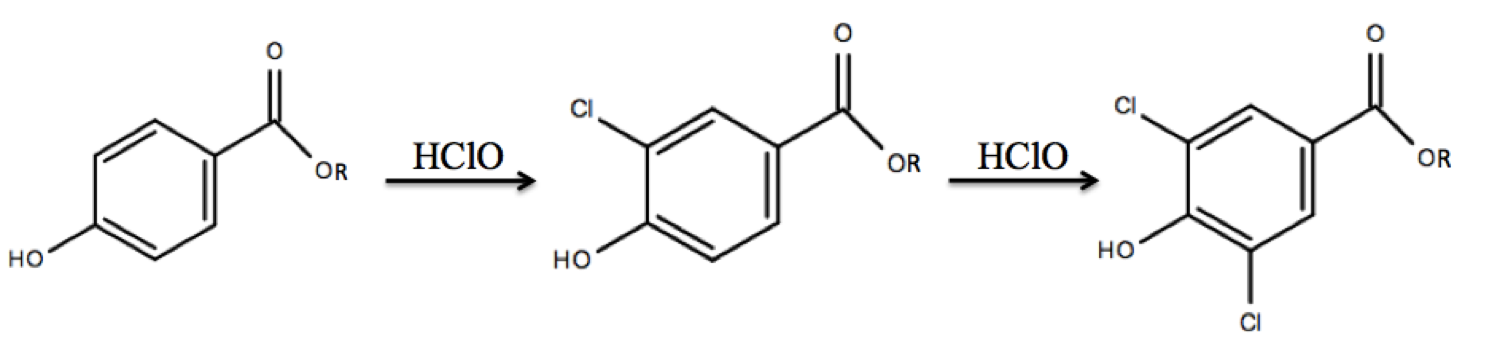
Chlorinated products


 In addition to parent parabens, paraben degradation products that form throughout WWTP stages present a concern to the environment, including mono- and di- chlorinated parabens. When paraben-containing products are washed down the drain, parabens have the potential to undergo chlorination reactions.Mao Q., Ji F., Wang W., Wang Q., Hu Z., Yuan S. (2016) Chlorination of parabens: reaction kinetics and transformation product identification. Environ. Sci. Polut. Res. 23, 23081–23091. This reaction can occur with free chlorine present in tap water or with
In addition to parent parabens, paraben degradation products that form throughout WWTP stages present a concern to the environment, including mono- and di- chlorinated parabens. When paraben-containing products are washed down the drain, parabens have the potential to undergo chlorination reactions.Mao Q., Ji F., Wang W., Wang Q., Hu Z., Yuan S. (2016) Chlorination of parabens: reaction kinetics and transformation product identification. Environ. Sci. Polut. Res. 23, 23081–23091. This reaction can occur with free chlorine present in tap water or with sodium hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of ...
, which is often used in WWTPs as a final disinfectant step.Terasaki M., Takemura Y., Makino M. (2012). Paraben-chlorinated derivatives are found in river water. Environ Chem Lett 10: 401–406 In neutral water, Raman spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman s ...
has confirmed that chlorine is predominantly present as hypochlorous acid
Hypochlorous acid (HClO, HOCl, or ClHO) is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine so ...
(HClO). Parabens can react with HClO to form mono- and di- chlorinated products through electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
. The electrophilic attack of the chlorine forms a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encoun ...
that is stabilized by donated electron density from the hydroxyl group of the paraben.Gowda B. T., Mary M. C. (2001) Kinetics and mechanism of chlorination of phenol and substituted phenols by sodium hypochlorite in aqueous alkaline medium. Indian Journal of Chemistry. 40, 1196–1202. This step is endergonic
In chemical thermodynamics, an endergonic reaction (; also called a heat absorbing nonspontaneous reaction or an unfavorable reaction) is a chemical reaction in which the standard change in free energy is positive, and an additional driving fo ...
due to the loss of aromaticity, though the hydroxyl group acts as an activating group that increases the rate. A base can then abstract
Abstract may refer to:
* ''Abstract'' (album), 1962 album by Joe Harriott
* Abstract of title a summary of the documents affecting title to parcel of land
* Abstract (law), a summary of a legal document
* Abstract (summary), in academic publishi ...
a proton from the carbon containing the chlorine, which is followed by subsequent restoration of aromaticity by the involved pi electrons
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbital ...
. Since the hydroxyl group is more activating than the ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...
group of the paraben, the reaction will direct in both ortho positions, as the para position is already blocked.
The Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 18 ...
was used in a study to calculate activation energies for the chlorination of four parent parabens (methyl-, ethyl-, propyl-, and butylparaben) and was found to range from 36–47kJ/mol. In another study, tap water at containing 50–200μM free chlorine was spiked with 0.5μM propylparaben and the composition of the mixture was monitored over 40 minutes to determine if chlorination occurs under conditions found in tap water. Results from the study confirm the disappearance of propylparaben after 5 minutes, the appearance of both 3-chloro-propylparaben and 3,5-dichloro-propylparaben paraben by 5 minutes, and the persistence of 3,5-dichloro-propylparaben as the main species remaining in the reaction. A similar, though more rapid, trend was found in a study in which the reaction temperature was increased to 35°C.
4-Hydroxybenzoic acid (PHBA)

 Another significant paraben degradation product is
Another significant paraben degradation product is 4-hydroxybenzoic acid
4-Hydroxybenzoic acid, also known as ''p''-hydroxybenzoic acid (PHBA), is a monohydroxybenzoic acid, a phenolic derivative of benzoic acid. It is a white crystalline solid that is slightly soluble in water and chloroform but more soluble in polar ...
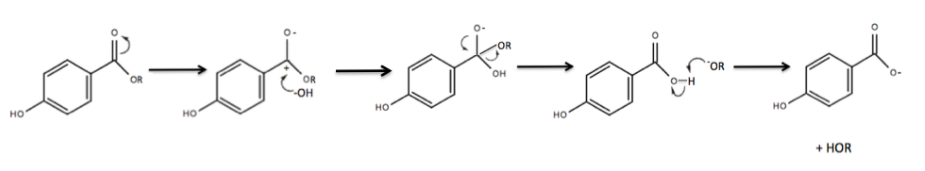
(PHBA). There are two mechanisms in which parabens can degrade to PHBA. The first degradation route occurs chemically. Parent parabens readily undergo base-catalyzed hydrolysis of the ester bond, forming PHBA. The reaction occurs under moderately alkaline conditions, specifically when the pH is ≥ 8. This reaction is quite prevalent in household environments due to the pH range of household wastewater being 6–9 and the prevalent existence of parabens in cosmetic products. When paraben-containing cosmetic products are discharged into community wastewater influent, they become exposed to an environment where the pH ≥ 8, and the base-catalyzed hydrolysis of the parent paraben ensues, forming PHBA.
In the electron transfer mechanism, the pi electrons in the double bond between the oxygen and carbonyl carbon resonate to the oxygen, leaving a negative charge on the oxygen and a positive charge on the carbonyl carbon. A hydroxide ion, acting as a nucleophile, attacks the now electrophilic carbonyl carbon, yielding sp3 hybridization on the carbonyl carbon. The electrons resonate back to form the double bond between the oxygen and the carbonyl carbon. In order to retain the original sp2 hybridization, the –OR group will leave. The –OR group acts as a better leaving group than the –OH group due to its ability to maintain a negative charge with greater stability. Lastly, the –OR-, acting as a base, will deprotonate the carboxylic acid to form a carboxylate anion.
The second way in which parabens can degrade into PHBA occurs biologically within WWTPs. During the secondary clarifier phase of Wastewater treatment
Wastewater treatment is a process used to remove contaminants from wastewater and convert it into an effluent that can be returned to the water cycle. Once returned to the water cycle, the effluent creates an acceptable impact on the environme ...
, sludge accumulates at the bottom of the secondary clarifier. Upon separation of the liquid and solid phases of the incoming influent, parabens have a greater tendency accumulate in the sludge. This is due to its moderate hydrophobicity, as quantified by a log Kow value of approximately 1.58. This sludge is concentrated in organic nutrients; consequently, a proliferation of microorganisms becomes common within the sludge. One organism is ''Enterobacter cloacae
''Enterobacter cloacae'' is a clinically significant Gram-negative, facultatively-anaerobic, rod-shaped bacterium.
Microbiology
In microbiology labs, ''E. cloacae'' is frequently grown at 30 °C on nutrient agar or at 35 °C in try ...
'', which biologically metabolizes the sludge parabens into PHBA.
Accumulation of degradation products in the environment
Through various analytical techniques such asgas chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, ...
and high-performance liquid chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa ...
, the exact levels of accumulation of paraben derivatives and degradation products in the environment have been quantified. These levels have been accurately measured in tertiary effluent and sewage sludge, as these are the primary avenues for which parabens and their degradation products reach the environment upon discharge from WWTPs.
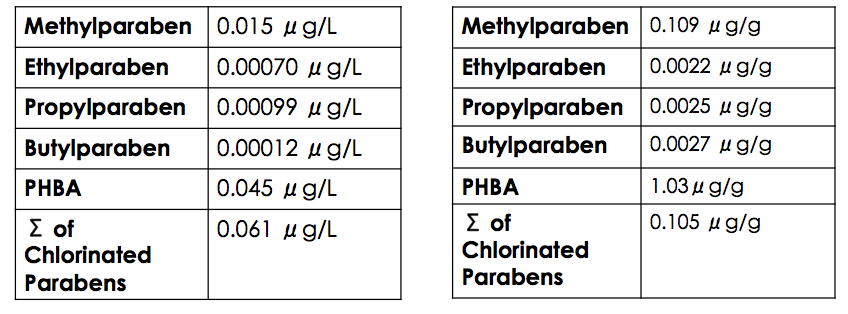
 Paraben stability in sewage sludge is relatively high due to their ability to bind with organic matter. Soil adsorption coefficient values were calculated by the U.S. Environmental Protection Agency as 1.94 (methylparaben), 2.20 (ethylparaben), 2.46 (propylparaben), and 2.72 (butylparaben), all of which suggest that parabens have the ability to adhere to the organic portion of sediment and sludge, and thus, persist environmentally.
Chlorinated parabens are removed from WWTPs with only 40% efficiency in comparison to 92–98% efficiency of parent parabens. The decrease in removal efficiency can be attributed to the decreased
Paraben stability in sewage sludge is relatively high due to their ability to bind with organic matter. Soil adsorption coefficient values were calculated by the U.S. Environmental Protection Agency as 1.94 (methylparaben), 2.20 (ethylparaben), 2.46 (propylparaben), and 2.72 (butylparaben), all of which suggest that parabens have the ability to adhere to the organic portion of sediment and sludge, and thus, persist environmentally.
Chlorinated parabens are removed from WWTPs with only 40% efficiency in comparison to 92–98% efficiency of parent parabens. The decrease in removal efficiency can be attributed to the decreased biodegradability
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegradati ...
of chlorinated parabens, their increased overall stability throughout WWTPs, and their relatively low sorption to the sludge phase due to low log Kow values.
Higher levels of PHBA are found in tertiary effluent in comparison to paraben derivatives, and PHBA exists in the highest concentration in sewage sludge. There are two reasons for these levels of accumulation. The first reason is PHBA's tendency to sorb to solid particles, which can be approximated by benzoic acid's high Kd value of approximately 19. The pKa of PHBA is 2.7, but it is in an environment of a pH between 6–9.Harashit M. (2014) Influence of Wastewater PH on Turbidity. International Journal of Environmental Research and Development. 4, 105–114. Since the pKa is less than the pH, the carboxylic acid will be deprotonated. The carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an ion with negative charge.
Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,...; ''carbox ...
allows it to act as a sorbent on solid environmental matrices, thus promoting its aggregation in tertiary effluent, but especially sewage sludge, which acts as the solid matrix itself. The second reason is due to the intermediate increase in levels of PHBA during the secondary clarifier phase of the WWTP through biological processes.
Environmental concerns with paraben degradation products
Multiple studies have linked chlorinated parabens to endocrine disrupting functions, specifically mimicking the effects ofestrogen
Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal a ...
, and chlorinated parabens are believed to be 3–4 times more toxic than their parent paraben.Terasaki M., Makino M., Tatarazako N. (2009) Acute toxicity of parabens and their chlorinated by-products with Daphnia magna and Vibrio fischeri bioassays. J. Appl. Toxicol. 29, 242–247. In ''Daphnia magna
''Daphnia magna'' is a small planktonic crustacean (adult length 1.5–5.0 mm) that belongs to the subclass Phyllopoda.
Description
''D. magna'' is a typical water flea of the genus ''Daphnia''. The females reach up to 5 mm in size, ...
'', general toxicity conferred by chlorinated parabens occurs through non-specific disruption of cell membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the ...
function. The potency of the chlorinated parabens correlates with the propensity of the compound to accumulate in cell membranes. Thus, chlorinated parabens generally increase in toxicity as their ester chains increase in length due to their increased hydrophobicity.
The implications of PHBA's environmental accumulation also warrants attention. If the tertiary effluent is re-used for community use as greywater
Greywater (or grey water, sullage, also spelled gray water in the United States) refers to domestic wastewater generated in households or office buildings from streams without fecal contamination, i.e., all streams except for the wastewater from ...
, it poses a hazard to humans. These hazards include, but are not limited to, abnormal fetal development, endocrine disrupting activity, and improper estrogen-promoting effects. If the tertiary effluent is released to the environment in rivers and streams or if the sludge is used as fertilizer, it poses as a hazard to environmental organisms. It is especially toxic to those organisms on lower trophic levels, particularly various algal species. In fact, it has been shown that the LC for a specific algal species, ''Selenastrum capricornutum
''Raphidocelis subcapitata'', formerly known as ''Selenastrum capricornutum'' and ''Pseudokirchneriella subcapitata'' is a microalga. This microalga presents a curved and twisted appearance like a sickle. The cells are normally presented in a s ...
'', is 0.032 micrograms per litre (μg/L).4-HYDROXYBENZOIC ACID. SIDS Initial Assessment Report for 9th SIAM, UNEP, 1999, France. This is less than the natural abundance of PHBA in tertiary effluent at a level of 0.045μg/L, thus indicating that current levels of PHBA in tertiary effluent can potentially eradicate more than 50% of ''Selenastrum capricornutum'' it comes in contact with.
Removal of parabens through ozonation
 Ozonation is an advanced treatment technique that has been considered as a possible method to limit the amount of parabens, chlorinated parabens, and PHBA that are accumulating in the environment. Ozone is an extremely powerful oxidant that oxidizes parabens and makes them easier to remove once subsequently passed through a filter.Tay K. S., Rahman N. A., Abas M. R. B. (2010) Ozonation of parabens in aqueous solutions: kinetics and mechanism of degradation. Chemosphere. 81, 1446–1453. Due to the electrophilic nature of ozone, it can easily react with the aromatic paraben ring to form hydroxylated products. Ozonation is generally regarded as a less dangerous method of disinfection than chlorination, though ozonation requires more cost considerations. Ozonation has demonstrated great efficacy in the removal of parabens (98.8–100%) and a slightly lower efficacy of 92.4% for PHBA. A moderately lower rate of removal, however, is observed for chlorinated parabens (59.2–82.8%). A proposed reaction mechanism for the removal of parabens by ozonation is detailed mechanistically.
Ozonation is an advanced treatment technique that has been considered as a possible method to limit the amount of parabens, chlorinated parabens, and PHBA that are accumulating in the environment. Ozone is an extremely powerful oxidant that oxidizes parabens and makes them easier to remove once subsequently passed through a filter.Tay K. S., Rahman N. A., Abas M. R. B. (2010) Ozonation of parabens in aqueous solutions: kinetics and mechanism of degradation. Chemosphere. 81, 1446–1453. Due to the electrophilic nature of ozone, it can easily react with the aromatic paraben ring to form hydroxylated products. Ozonation is generally regarded as a less dangerous method of disinfection than chlorination, though ozonation requires more cost considerations. Ozonation has demonstrated great efficacy in the removal of parabens (98.8–100%) and a slightly lower efficacy of 92.4% for PHBA. A moderately lower rate of removal, however, is observed for chlorinated parabens (59.2–82.8%). A proposed reaction mechanism for the removal of parabens by ozonation is detailed mechanistically.
References
{{Reflist