Imene Merrouche on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 :A nitrene intermediate is suspected in this C–H insertion involving an oxime,
:A nitrene intermediate is suspected in this C–H insertion involving an oxime,  * Nitrene cycloaddition. With alkenes, nitrenes react to form aziridines, very often with nitrenoid precursors such as nosyl- or tosyl-substituted 'N''-(phenylsulfonyl)iminohenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the
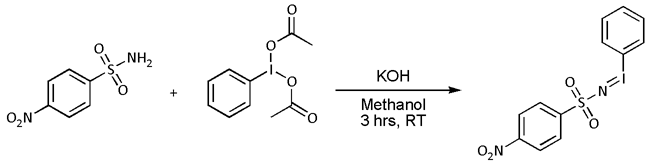
* Nitrene cycloaddition. With alkenes, nitrenes react to form aziridines, very often with nitrenoid precursors such as nosyl- or tosyl-substituted 'N''-(phenylsulfonyl)iminohenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the  :In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
::
:In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
:: :Nitrene transfer takes place next:
::
:Nitrene transfer takes place next:
:: :In this particular reaction both the ''
:In this particular reaction both the ''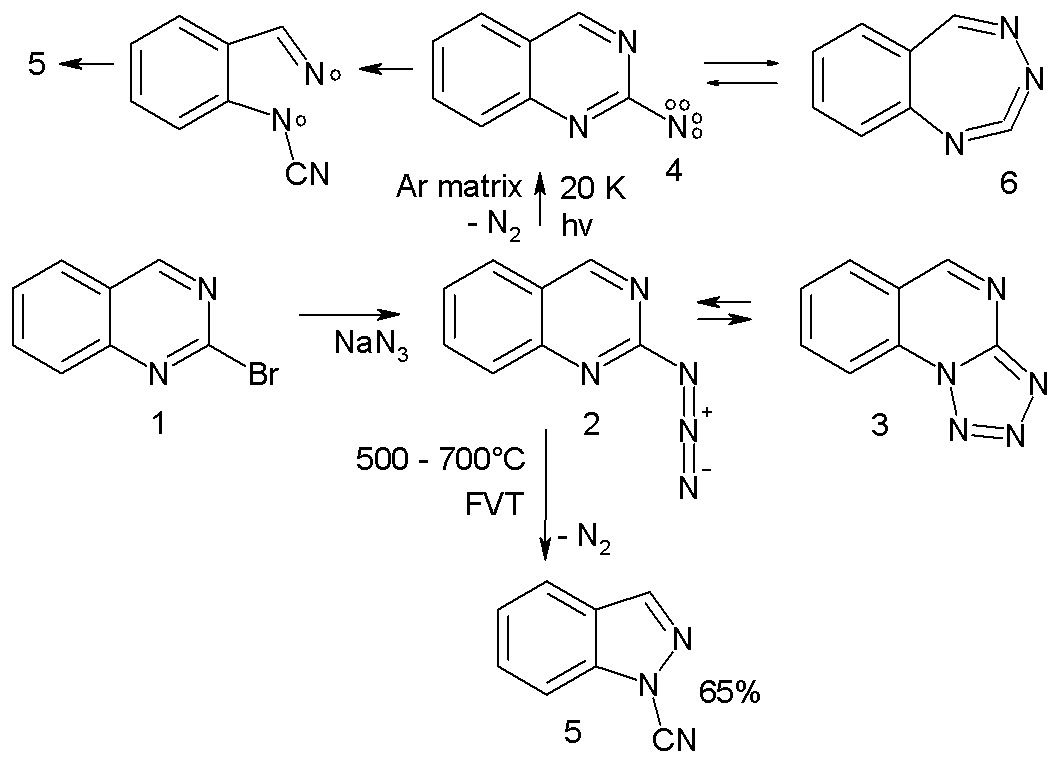 :The nitrene ultimately converts to the ring-opened
:The nitrene ultimately converts to the ring-opened
 In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a σ–σ–π triradical. A carbene nitrogen radical (imidyl radical) resonance structure makes a contribution to the total electronic picture.
In 2019, an authentic triplet nitrene was isolated by Betley and Lancaster, stabilized by coordination to a copper center in a bulky ligand.
In this system one of the nitrogen unpaired electrons is delocalized in the aromatic ring making the compound a σ–σ–π triradical. A carbene nitrogen radical (imidyl radical) resonance structure makes a contribution to the total electronic picture.
In 2019, an authentic triplet nitrene was isolated by Betley and Lancaster, stabilized by coordination to a copper center in a bulky ligand.
 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, a nitrene or imene () is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
ed and four non-bonded electrons. It is therefore considered an electrophile due to the unsatisfied octet. A nitrene is a reactive intermediate
In chemistry, a reactive intermediate or an intermediate is a short-lived, high-energy, highly reactive molecule. When generated in a chemical reaction, it will quickly convert into a more stable molecule. Only in exceptional cases can these comp ...
and is involved in many chemical reactions. The simplest nitrene, HN, is called imidogen, and that term is sometimes used as a synonym for the nitrene class.
Electron configuration
In the simplest case, the linear N–H molecule (imidogen) has its nitrogen atom sp hybridized, with two of its four non-bonded electrons as alone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
in an sp orbital and the other two occupying a degenerate
Degeneracy, degenerate, or degeneration may refer to:
Arts and entertainment
* ''Degenerate'' (album), a 2010 album by the British band Trigger the Bloodshed
* Degenerate art, a term adopted in the 1920s by the Nazi Party in Germany to descr ...
pair of p orbitals. The electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
is consistent with Hund's rule: the low energy form is a triplet
A triplet is a set of three items, which may be in a specific order, or unordered. It may refer to:
Science
* A series of three nucleotide bases forming an element of the Genetic code
* J-coupling as part of Nuclear magnetic resonance spectrosc ...
with one electron in each of the p orbitals and the high energy form is the singlet with an electron pair filling one p orbital and the other p orbital vacant.
As with carbenes, a strong correlation exists between the spin density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
on the nitrogen atom which can be calculated in silico
In biology and other experimental sciences, an ''in silico'' experiment is one performed on computer or via computer simulation. The phrase is pseudo-Latin for 'in silicon' (correct la, in silicio), referring to silicon in computer chips. It ...
and the zero-field splitting parameter
Zero field splitting (ZFS) describes various interactions of the energy levels of a molecule or ion resulting from the presence of more than one unpaired electron. In quantum mechanics, an energy level is called degenerate if it corresponds to two ...
''D'' which can be derived experimentally from electron spin resonance. Small nitrenes such as NH or CF3N have D values around 1.8 cm−1 with spin densities close to a maximum value of 2. At the lower end of the scale are molecules with low ''D'' (< 0.4) values and spin density of 1.2 to 1.4 such as 9-anthrylnitrene and 9-phenanthrylnitrene.
Formation
Because nitrenes are so reactive, they are not isolated. Instead, they are formed as reactive intermediates during a reaction. There are two common ways to generate nitrenes: * Fromazide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
s by thermolysis or photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by photons. It is defined as the interaction of one or more photons with one target molecule. ...
, with expulsion of nitrogen gas. This method is analogous to the formation of carbenes from diazo compounds.
* From isocyanates, with expulsion of carbon monoxide. This method is analogous to the formation of carbenes from ketenes.
Reactions
Nitrene reactions include: * Nitrene C–H insertion. A nitrene can easily insert into a carbon to hydrogencovalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
yielding an amine or amide. A singlet nitrene reacts with retention of configuration
Walden inversion is the inversion of a stereogenic center in a chiral molecule in a chemical reaction. Since a molecule can form two enantiomers around a stereogenic center, the Walden inversion converts the configuration of the molecule from ...
. In one study a nitrene, formed by oxidation of a carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
with potassium persulfate, gives an insertion reaction into the palladium to nitrogen bond of the reaction product of palladium(II) acetate with 2-phenylpyridine
2-Phenylpyridine is an organic compound with the formula C6H5C5H4N (or C11H9N). It is a colourless viscous liquid. The compound and related derivatives have attracted interest as precursors to highly fluorescent metal complexes of possible value ...
to methyl ''N''-(2-pyridylphenyl)carbamate in a cascade reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the p ...
:
:: :A nitrene intermediate is suspected in this C–H insertion involving an oxime,
:A nitrene intermediate is suspected in this C–H insertion involving an oxime, acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a col ...
leading to an isoindole:
:: * Nitrene cycloaddition. With alkenes, nitrenes react to form aziridines, very often with nitrenoid precursors such as nosyl- or tosyl-substituted 'N''-(phenylsulfonyl)iminohenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the
* Nitrene cycloaddition. With alkenes, nitrenes react to form aziridines, very often with nitrenoid precursors such as nosyl- or tosyl-substituted 'N''-(phenylsulfonyl)iminohenyliodinane (PhI=NNs or PhI=NTs respectively)) but the reaction is known to work directly with the sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. ...
in presence of a transition metal based catalyst such as copper, palladium, or gold:
:: :In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
::
:In most cases, however, 'N''-(''p''-nitrophenylsulfonyl)iminohenyliodinane (PhI=NNs) is prepared separately as follows:
:: :Nitrene transfer takes place next:
::
:Nitrene transfer takes place next:
:: :In this particular reaction both the ''
:In this particular reaction both the ''cis
Cis or cis- may refer to:
Places
* Cis, Trentino, in Italy
* In Poland:
** Cis, Świętokrzyskie Voivodeship, south-central
** Cis, Warmian-Masurian Voivodeship, north
Math, science and biology
* cis (mathematics) (cis(''θ'')), a trigonome ...
''- stilbene illustrated and the ''trans'' form (not depicted) result in the same ''trans''-aziridine product, suggesting a two-step reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of ...
. The energy difference between triplet and singlet nitrenes can be very small in some cases, allowing interconversion at room temperature. Triplet nitrenes are thermodynamically more stable but react stepwise allowing free rotation and thus producing a mixture of stereochemistry.
* Arylnitrene ring-expansion and ring-contraction: Aryl nitrenes show ring expansion to 7-membered ring cumulenes, ring opening reactions and nitrile formations many times in complex reaction paths. For instance the azide 2 in the scheme below trapped in an argon matrix
Matrix most commonly refers to:
* ''The Matrix'' (franchise), an American media franchise
** '' The Matrix'', a 1999 science-fiction action film
** "The Matrix", a fictional setting, a virtual reality environment, within ''The Matrix'' (franchi ...
at 20 K on photolysis expels nitrogen to the triplet nitrene 4 (observed experimentally with ESR and ultraviolet-visible spectroscopy) which is in equilibrium with the ring-expansion product 6.
: :The nitrene ultimately converts to the ring-opened
:The nitrene ultimately converts to the ring-opened nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
5 through the diradical intermediate 7. In a high-temperature reaction, FVT at 500–600 °C also yields the nitrile 5 in 65% yield.
Nitreno radicals
For several compounds containing both a nitrene group and a free radical group an ESR high-spin quartet has been recorded (matrix, cryogenic temperatures). One of these has an amine oxide radical group incorporated, another system has a carbon radical group. :References
{{Functional group Reactive intermediates Free radicals Functional groups Nitrogen compounds