Hinsberg Test on:
[Wikipedia]
[Google]
[Amazon]
The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary
 For primary amines (R' = H), the initially formed sulfonamide is deprotonated by base to give a water-soluble sulfonamide salt (Na hSO2NR.
:PhSO2N(H)R + NaOH → Na+ hSO2NR−+ H2O
For primary amines (R' = H), the initially formed sulfonamide is deprotonated by base to give a water-soluble sulfonamide salt (Na hSO2NR.
:PhSO2N(H)R + NaOH → Na+ hSO2NR−+ H2O
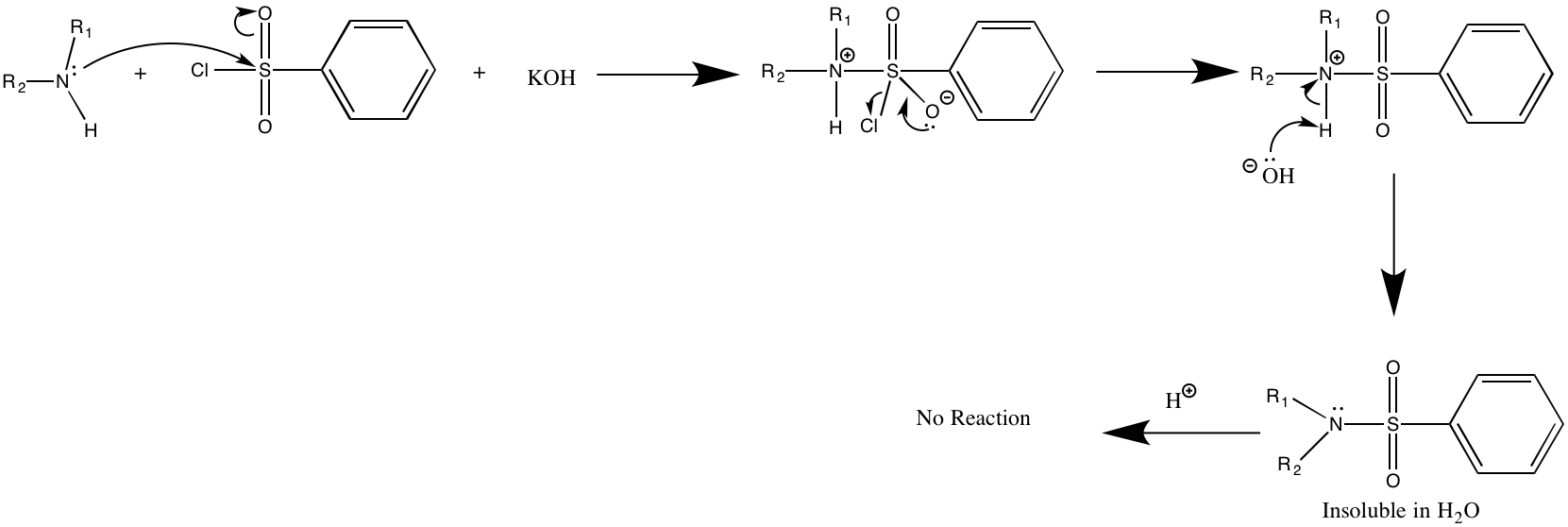 Tertiary amines promote hydrolysis of the sulfonyl chloride functional group, which affords water-soluble sulfonate salts.
:PhSO2Cl + R3N + H2O → R3NH+ hSO+ HCl
Tertiary amines promote hydrolysis of the sulfonyl chloride functional group, which affords water-soluble sulfonate salts.
:PhSO2Cl + R3N + H2O → R3NH+ hSO+ HCl

science.csustan.edu
Chemical tests
amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with Hinsberg reagent in the presence of aqueous alkali (either KOH or NaOH). A reagent containing an aqueous sodium hydroxide solution and benzenesulfonyl chloride
Benzenesulfonyl chloride is an organosulfur compound with the formula C6H5SO2Cl. It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds. It is mainly used to prepare sulfo ...
is added to a substrate. A primary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent(benzene sulfonyl chloride) and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ...
. Hinsberg test Is Used to distinguish between 1,2,3 degree amine with help of sulphonly cloride
In this way the reaction can distinguish between the three types of amines.
Tertiary amines are able to react with benzenesulfonyl chloride under a variety of conditions; the test described above is not absolute. The Hinsberg test for amines is valid only when reaction speed, concentration, temperature, and solubility are taken into account.
Reactions
Amines serve as nucleophiles in attacking the sulfonyl chloride electrophile, displacing chloride. The sulfonamides resulting from primary and secondary amines are poorly soluble and precipitate as solids from solution. :PhSO2Cl + 2 RR'NH → PhSO2NRR' + R'NH2+l− For primary amines (R' = H), the initially formed sulfonamide is deprotonated by base to give a water-soluble sulfonamide salt (Na hSO2NR.
:PhSO2N(H)R + NaOH → Na+ hSO2NR−+ H2O
For primary amines (R' = H), the initially formed sulfonamide is deprotonated by base to give a water-soluble sulfonamide salt (Na hSO2NR.
:PhSO2N(H)R + NaOH → Na+ hSO2NR−+ H2O
 Tertiary amines promote hydrolysis of the sulfonyl chloride functional group, which affords water-soluble sulfonate salts.
:PhSO2Cl + R3N + H2O → R3NH+ hSO+ HCl
Tertiary amines promote hydrolysis of the sulfonyl chloride functional group, which affords water-soluble sulfonate salts.
:PhSO2Cl + R3N + H2O → R3NH+ hSO+ HCl

References
External links
{{cc * Laboratory procedurescience.csustan.edu
Chemical tests