|
Hinsberg Test
The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with Hinsberg reagent in the presence of aqueous alkali (either KOH or NaOH). A reagent containing an aqueous sodium hydroxide solution and benzenesulfonyl chloride is added to a substrate. A primary amine will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent(benzene sulfonyl chloride) and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for pos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oscar Hinsberg
Oscar Heinrich Daniel Hinsberg (21 October 1857 – 13 February 1939) was a German chemist. Hinsberg was born in Berlin. In 1882 he obtained his doctorate in sciences at the University of Tübingen, later serving as a professor at the Universities of Freiburg and Geneva. He is known for research involving synthesis of oxindole, sulfone and thiophene. In 1890 he introduced the "Hinsberg reaction", a test used for differentiation of primary, secondary and tertiary amines. Publications * ''Ueber Oxalsäurederivate des Metanitroparatoluidins und des Metaparadiamidotoluols'', 1882. * ''Ueber die Wirkung des Acetphenetidins'', (with internist Alfred Kast 1856-1903). in Centralblatt für die medicinischen Wissenschaften, Berlin, 1887, 25: 145-148. - introduction of phenacetine. @ |
Benzenesulfonyl Chloride
Benzenesulfonyl chloride is an organosulfur compound with the formula C6H5SO2Cl. It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds. It is mainly used to prepare sulfonamides and sulfonate esters by reactions with amines and alcohols, respectively. The closely related compound toluenesulfonyl chloride is often preferred analogue because it is a solid at room temperature and easier to handle. The compound is prepared by the chlorination of benzenesulfonic acid or its salts with phosphorus oxychloride or, less commonly, by a reaction between benzene and chlorosulfuric acid. The Hinsberg test The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with Hinsberg reagent in the presence of aque ... for amines involves their reaction with benzenesulfonyl chlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions . Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burns. It is highly soluble in water, and readily absorbs moisture and carbon dioxide from the air. It forms a series of hydrates . The monohydrate crystallizes from water solutions between 12.3 and 61.8 °C. The commercially available "sodium hydroxide" is often this monohydrate, and published data may refer to it instead of the anhydrous compound. As one of the simplest hydroxides, sodium hydroxide is frequently used alongside neutral water and acidic hydrochloric acid to demonstrate the pH scale to chemistry students. Sodium hydroxide is used in many industries: in the manufacture of pulp and paper, textiles, drinking water, soaps and deterge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenesulfonyl Chloride
Benzenesulfonyl chloride is an organosulfur compound with the formula C6H5SO2Cl. It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds. It is mainly used to prepare sulfonamides and sulfonate esters by reactions with amines and alcohols, respectively. The closely related compound toluenesulfonyl chloride is often preferred analogue because it is a solid at room temperature and easier to handle. The compound is prepared by the chlorination of benzenesulfonic acid or its salts with phosphorus oxychloride or, less commonly, by a reaction between benzene and chlorosulfuric acid. The Hinsberg test The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with Hinsberg reagent in the presence of aque ... for amines involves their reaction with benzenesulfonyl chlo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
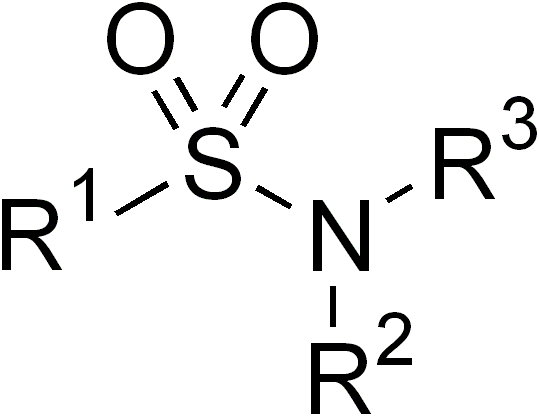
Sulfonamide (chemistry)
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Salt
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |