Hydrogen Electrolysis on:
[Wikipedia]
[Google]
[Amazon]

 Electrolysis of water, also known as electrochemical
Electrolysis of water, also known as electrochemical
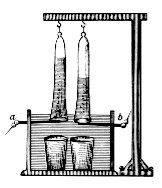 In 1789, Jan Rudolph Deiman and Adriaan Paets van Troostwijk used an electrostatic machine to make electricity that was discharged on gold electrodes in a
In 1789, Jan Rudolph Deiman and Adriaan Paets van Troostwijk used an electrostatic machine to make electricity that was discharged on gold electrodes in a
 In pure water at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas. The
In pure water at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas. The
 The decomposition of pure water into hydrogen and oxygen at
The decomposition of pure water into hydrogen and oxygen at
 Efficiency of modern hydrogen generators is measured by ''energy consumed per standard volume of hydrogen'' (MJ/m3), assuming
Efficiency of modern hydrogen generators is measured by ''energy consumed per standard volume of hydrogen'' (MJ/m3), assuming
Archived
10 March 2016 at the Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–80%, producing 1 kg of hydrogen (which has a
Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–80%, producing 1 kg of hydrogen (which has a
Hydrogen Production Tech Team Roadmap, November 2017
putting the cost of steam-methane-reformed (SMR) hydrogen at between $1.20 and $1.50, the cost price of hydrogen via electrolysis is still over double 2015 DOE hydrogen target prices. The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kW·h, which is achievable given 2018 PPA tenders for wind and solar in many regions. This puts the $4/gasoline gallon equivalent (gge) H2 dispensed objective well within reach, and close to a slightly elevated natural gas production cost for SMR. In other parts of the world, the price of SMR hydrogen is between $1–3/kg on average. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen and others, including an article by the IEA examining the conditions which could lead to a competitive advantage for electrolysis. The large price increase of gas during the 2021–2022 global energy crisis made hydrogen electrolysis economic in some parts of the world. Some large industrial electrolyzers are operating at several megawatts. , the largest is a 150 MW alkaline facility in Ningxia, China, with a capacity up to 23,000 tonnes per year. While higher-efficiency Western electrolysis equipment can cost $1,200/kW, lower-efficiency Chinese equipment can cost $300/kW, but with a lower lifetime of 60,000 hours.
EERE 2008 – 100 kgH2/day Trade StudyNREL 2006 – Electrolysis technical report
{{DEFAULTSORT:Electrolysis Of Water Electrolysis Hydrogen production Industrial gases Water chemistry
 Electrolysis of water, also known as electrochemical
Electrolysis of water, also known as electrochemical water splitting
Water splitting is the chemical reaction in which water is broken down into oxygen and hydrogen:
:2 H2O → 2 H2 + O2
Efficient and economical water splitting would be a technological breakthrough that could underpin a hydrogen economy, base ...
, is the process of using electricity to decompose water into oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
gas by electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
. Hydrogen gas released in this way can be used as hydrogen fuel
Hydrogen fuel refers to hydrogen which is burned as fuel with oxygen. It is zero-carbon, provided that it is created in a process that does not involve carbon. It can be used in fuel cells or internal combustion engines (see HICEV). Regarding hydr ...
, or remixed with the oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
to create oxyhydrogen
Oxyhydrogen is a mixture of hydrogen (H2) and oxygen (O2) gases. This gaseous mixture is used for torches to process refractory materials and was the first
gaseous mixture used for welding. Theoretically, a ratio of 2:1 hydrogen:oxygen is enough ...
gas, which is used in welding and other applications.
Electrolysis of water requires a minimum potential difference
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to m ...
of 1.23 volt
The volt (symbol: V) is the unit of electric potential, electric potential difference (voltage), and electromotive force in the International System of Units (SI). It is named after the Italian physicist Alessandro Volta (1745–1827).
Defi ...
s, though at that voltage external heat is required.
E lectrolysis is rarely used in industrial applications since hydrogen can be produced less expensively from fossil fuel
A fossil fuel is a hydrocarbon-containing material formed naturally in the Earth's crust from the remains of dead plants and animals that is extracted and burned as a fuel. The main fossil fuels are coal, oil, and natural gas. Fossil fuels m ...
s.
History
 In 1789, Jan Rudolph Deiman and Adriaan Paets van Troostwijk used an electrostatic machine to make electricity that was discharged on gold electrodes in a
In 1789, Jan Rudolph Deiman and Adriaan Paets van Troostwijk used an electrostatic machine to make electricity that was discharged on gold electrodes in a Leyden jar
A Leyden jar (or Leiden jar, or archaically, sometimes Kleistian jar) is an electrical component that stores a high-voltage electric charge (from an external source) between electrical conductors on the inside and outside of a glass jar. It typi ...
with water. In 1800 Alessandro Volta
Alessandro Giuseppe Antonio Anastasio Volta (, ; 18 February 1745 – 5 March 1827) was an Italian physicist, chemist and lay Catholic who was a pioneer of electricity and power who is credited as the inventor of the electric battery and the ...
invented the voltaic pile
upright=1.2, Schematic diagram of a copper–zinc voltaic pile. The copper and zinc discs were separated by cardboard or felt spacers soaked in salt water (the electrolyte). Volta's original piles contained an additional zinc disk at the bottom, ...
, and a few weeks later English scientists William Nicholson and Anthony Carlisle
Sir Anthony Carlisle FRCS, FRS (15 February 1768 in Stillington, County Durham, England – 2 November 1840 in London) was an English surgeon.
Life
He was born in Stillington, County Durham, the third son of Thomas Carlisle and his first wife, ...
used it to electrolyse water. In 1806 Humphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
reported the results of extensive distilled water electrolysis experiments, concluding that nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available nitri ...
was produced at the anode from dissolved atmospheric nitrogen gas. He used a high voltage battery and non-reactive electrodes and vessels such as gold electrode cones that doubled as vessels bridged by damp asbestos. Zénobe Gramme
Zénobe Théophile Gramme (4 April 1826 – 20 January 1901) was a Belgian electrical engineer. He was born at Jehay-Bodegnée on 4 April 1826, the sixth child of Mathieu-Joseph Gramme, and died at Bois-Colombes on 20 January 1901. He invented ...
invented the Gramme machine
A Gramme machine, Gramme ring, Gramme magneto, or Gramme dynamo is an electrical generator that produces direct current, named for its Belgian inventor, Zénobe Gramme, and was built as either a dynamo or a magneto. It was the first generator to p ...
in 1869, making electrolysis a cheap method for hydrogen production
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (∼95%) is produced from fossil fuels by steam reforming of natural gas and other light hydrocarbons, partial oxidation of h ...
. A method of industrial synthesis of hydrogen and oxygen through electrolysis was developed by Dmitry Lachinov
Dmitry Aleksandrovich Lachinov (russian: Дмитрий Александрович Лачи́нов ) (10 Jan
1842 – 15 October 1902) was a Russian physicist, electrical engineer, inventor, meteorologist and climatologist.
Biography
Dmitry ...
in 1888.
Principle
A DC electrical power source is connected to twoelectrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials de ...
s, or two plates (typically made from an inert metal such as platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
or iridium
Iridium is a chemical element with the symbol Ir and atomic number 77. A very hard, brittle, silvery-white transition metal of the platinum group, it is considered the second-densest naturally occurring metal (after osmium) with a density of ...
) that are placed in the water. Hydrogen appears at the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
(where electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s enter the water), and oxygen at the anode
An anode is an electrode of a polarized electrical device through which conventional current enters the device. This contrasts with a cathode, an electrode of the device through which conventional current leaves the device. A common mnemonic is ...
. Assuming ideal faradaic efficiency Faraday efficiency (also called ''faradaic efficiency'', ''faradaic yield'', ''coulombic efficiency'' or ''current efficiency'') describes the efficiency with which charge (electrons) is transferred in a system facilitating an electrochemical reacti ...
, the amount
Quantity or amount is a property that can exist as a multitude or magnitude, which illustrate discontinuity and continuity. Quantities can be compared in terms of "more", "less", or "equal", or by assigning a numerical value multiple of a unit ...
of hydrogen generated is twice the amount of oxygen, and both are proportional to the total electrical charge
Electricity is the set of physical phenomena associated with the presence and motion of matter that has a property of electric charge. Electricity is related to magnetism, both being part of the phenomenon of electromagnetism, as described by ...
conducted by the solution. However, in many cells competing side reactions occur, resulting in additional products and less than ideal faradaic efficiency.
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of pure water requires excess energy in the form of overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly relat ...
to overcome various activation barriers. Without the excess energy, electrolysis occurs very slowly or not at all. This is in part due to the limited self-ionization of water
The self-ionization of water (also autoionization of water, and autodissociation of water) is an ionization reaction in pure water or in an aqueous solution, in which a water molecule, H2O, deprotonates (loses the nucleus of one of its hydrogen ...
. Pure water has an electrical conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allow ...
about one-millionth that of seawater. Many electrolytic cell
An electrolytic cell is an electrochemical cell that utilizes an external source of electrical energy to force a chemical reaction that would not otherwise occur. The external energy source is a voltage applied between the cell′s two electrod ...
s lack the requisite electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst ...
s. Efficiency is increased through the addition of an electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
(such as a salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantitie ...
, an acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
or a base) and electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst ...
s.
Equations
 In pure water at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas. The
In pure water at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas. The half reaction
A half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction. ...
, balanced with acid, is:
:Reduction at cathode:
2H+('' aq'') + 2e− → H2('' g'')
At the positively charged anode, an oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
reaction occurs, generating oxygen gas and giving electrons to the anode to complete the circuit:
:Oxidation at anode: 2 H2O('' l'') → O2(''g'') + 4 H+(''aq'') + 4e−
The same half-reactions can also be balanced with the base as listed below. Not all half-reactions must be balanced with acid or base. Many do, like the oxidation or reduction of water listed here. To add half reactions they must both be balanced with either acid or base. The acid-balanced reactions predominate in acidic (low pH) solutions, while the base-balanced reactions predominate in basic (high pH) solutions.
Combining either half reaction pair yields the same overall decomposition of water into oxygen and hydrogen:
:Overall reaction: 2 H2O(''l'') → 2 H2(''g'') + O2(''g'')
The number of hydrogen molecules produced is thus twice the number of oxygen molecules. Assuming equal temperature and pressure for both gases, the produced hydrogen gas has, therefore, twice the volume of the produced oxygen gas. The number of electrons pushed through the water is twice the number of generated hydrogen molecules and four times the number of generated oxygen molecules.
Thermodynamics
 The decomposition of pure water into hydrogen and oxygen at
The decomposition of pure water into hydrogen and oxygen at standard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
is not favorable in thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of ther ...
terms.
Thus, the standard potential of the water electrolysis cell (Eocell = Eocathode − Eoanode) is -1.229 V at 25 °C at pH 0 ( += 1.0 M). At 25 °C with pH 7 ( += 1.0 M), the potential is unchanged based on the Nernst equation
In electrochemistry, the Nernst equation is a Thermodynamics#Chemical thermodynamics, chemical thermodynamical relationship that permits the calculation of the reduction potential of a reaction (half-cell or electrochemical cell, full cell reacti ...
. The thermodynamic standard cell potential can be obtained from standard-state free energy calculations to find ΔG° and then using the equation: ΔG°= −n F E° (where E° is the cell potential and F the Faraday constant
In physical chemistry, the Faraday constant, denoted by the symbol and sometimes stylized as ℱ, is the electric charge per mole of elementary charges. It is named after the English scientist Michael Faraday. Since the 2019 redefinition of S ...
, i.e. 96,485.3321233 C/mol). For two water molecules electrolysed and hence two hydrogen molecules formed, n = 4, and ΔG° = 474.48 kJ/2 mol(water) = 237.24 kJ/mol(water), and ΔS° = 163 J/K mol(water), and ΔH° = 571.66 kJ/2 mol(water) = 285.83 kJ/mol(water), and finally 141.86 MJ/kg(H2). However, calculations regarding individual electrode equilibrium potentials requires some corrections taking into account the activity coefficients. In practice when an electrochemical cell is "driven" toward completion by applying reasonable potential, it is kinetically controlled. Therefore, activation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules pe ...
, ion mobility (diffusion) and concentration, wire resistance, surface hindrance including bubble formation (causes electrode area blockage), and entropy, require a greater applied potential to overcome these factors. The amount of increase in potential required is termed the overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly relat ...
.
Electrolyte
Electrolysis in pure water consumes/reduces H+cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s at the cathode and consumes/oxidizes hydroxide (OH−) anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s at the anode. This can be verified by adding a pH indicator
A pH indicator is a halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically by changes in absorption and/or emission properties. Hence, ...
to the water: water near the cathode is basic while water near the anode is acidic. The hydroxides OH− that approach the anode mostly combine with the positive hydronium ions (H3O+) to form water. The positive hydronium ions that approach the cathode mostly combine with negative hydroxide ions to form water. Relatively few hydroniums/hydroxide ions reach the cathode/anode. This can cause overpotential at both electrodes.
Pure water has a charge carrier density similar to semiconductors since it has a low autoionization
Autoionization is a process by which an atom or a molecule in an excited state spontaneously emits one of the outer-shell electrons, thus going from a state with charge to a state with charge , for example from an electrically neutral st ...
, Kw = 1.0×10−14 at room temperature and thus pure water conducts current poorly, 0.055 µS·cm−1. Unless a large potential is applied to increase the autoionization of water electrolysis of pure water proceeds very slowly limited by the overall conductivity.
A water-soluble electrolyte can considerably raise conductivity. The electrolyte disassociates into cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s and anions; the anions rush towards the anode and neutralize the buildup of positively charged H+ there; similarly, the cations rush towards the cathode and neutralize the buildup of negatively charged OH− there. This allows the continuous flow of electricity.
Anions from the electrolyte compete with the hydroxide ions to give up an electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
. An electrolyte anion with less standard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as: ''"the value of the standard emf (electromotive force) of a cell in wh ...
than hydroxide will be oxidized instead of the hydroxide, producing no oxygen gas. Instead a cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
with a greater standard electrode potential
In electrochemistry, standard electrode potential E^\ominus, or E^\ominus_, is a measure of the reducing power of any element or compound. The IUPAC "Gold Book" defines it as: ''"the value of the standard emf (electromotive force) of a cell in wh ...
than a hydrogen ion is reduced instead, producing no hydrogen gas.
Various cations
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
have lower electrode potential
In electrochemistry, electrode potential is the electromotive force of a galvanic cell built from a standard reference electrode and another electrode to be characterized. By convention, the reference electrode is the standard hydrogen electrode (S ...
than H+ and are therefore suitable for use as electrolyte cations: Li+, Rb+, K+, Cs+, Ba2+, Sr2+, Ca2+, Na+, and Mg2+. Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable iso ...
and lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid el ...
are common choices, as they form inexpensive, soluble salts.
If an acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
is used as the electrolyte, the cation is H+, and no competitor for the H+ is created by disassociating water. The most commonly used anion is sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ar ...
(), as it is difficult to oxidize. The standard potential for oxidation of this ion to the peroxydisulfate
The peroxydisulfate ion, , is an oxyanion, the anion of peroxydisulfuric acid. It is commonly referred to as persulfate, but this term also refers to the peroxomonosulfate ion, . It is also called ''peroxodisulfate''. Approximately 500,000 tons o ...
ion is +2.010 volts.
Strong acids such as sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
(H2SO4), and strong bases such as potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which exp ...
(KOH), and sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
(NaOH) are common choices as electrolytes due to their strong conducting abilities.
A solid polymer electrolyte can be used such as Nafion
Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Dr. Walther Grot of DuPont. Nafion is a brand of the Chemours company. It is the first of a class of synthetic polymers with ...
and when applied with a special catalyst on each side of the membrane can efficiently split the water molecule with as little as 1.5 volts. Several commercial electrolysis systems use solid electrolytes.
Pure water electrolysis
Electrolyte-free pure water electrolysis has been achieved via deep-sub-Debye-length nanogapelectrochemical cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic o ...
s. When the gap between cathode and anode are smaller than Debye-length (1 micron in pure water, around 220 nm in distilled water), the double layer regions from two electrodes can overlap, leading to a uniformly high electric field distributed across the entire gap. Such a high electric field can significantly enhance ion transport (mainly due to migration), further enhancing self-ionization, continuing the reaction and showing little resistance between the two electrodes. In this case, the two half-reaction
A half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction. ...
s are coupled and limited by electron-transfer steps (the electrolysis current is saturated at shorter electrode distances).
Techniques
As of 2022, commercial electrolysis requires around 53 kWh of electricity to produce one kg of hydrogen, which holds 39.4 kWh of energy.Fundamental demonstration
Twoleads
Lead is a chemical element with symbol Pb and atomic number 82.
Lead or The Lead may also refer to:
Animal handling
* Leash, or lead
* Lead (leg), the leg that advances most in a quadruped's cantering or galloping stride
* Lead (tack), a lin ...
, running from the terminals of a battery, placed in a cup of water with a quantity of electrolyte establish conductivity. Using NaCl
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
(salt) in an electrolyte solution yields chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
gas rather than oxygen due to a competing half-reaction. Sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
(baking soda) instead yield hydrogen and oxygen gases.
Hofmann voltameter
The Hofmann voltameter is a small-scale electrolytic cell. It consists of three joined upright cylinders. The inner cylinder is open at the top to allow the addition of water and electrolyte. Aplatinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
electrode (plate or honeycomb) is placed at the bottom of each of the two side cylinders, connected to the terminals of an electricity source. The generated gases displace water and collect at the top of the two outer tubes, where it can be drawn off with a stopcock.
High-pressure
High-pressure electrolysis involvescompressed hydrogen
Compressed hydrogen (CH2, CGH2 or CGH2) is the gaseous state of the element hydrogen kept under pressure. Compressed hydrogen in hydrogen tanks at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for mobile hydrogen storage in hydrogen vehicles ...
output around 12–20 MPa (120–200 Bar
Bar or BAR may refer to:
Food and drink
* Bar (establishment), selling alcoholic beverages
* Candy bar
* Chocolate bar
Science and technology
* Bar (river morphology), a deposit of sediment
* Bar (tropical cyclone), a layer of cloud
* Bar (u ...
, 1740–2900 psi
Psi, PSI or Ψ may refer to:
Alphabetic letters
* Psi (Greek) (Ψ, ψ), the 23rd letter of the Greek alphabet
* Psi (Cyrillic) (Ѱ, ѱ), letter of the early Cyrillic alphabet, adopted from Greek
Arts and entertainment
* "Psi" as an abbreviatio ...
). By pressurising the hydrogen in the electrolyser, the need for an external hydrogen compressor
A hydrogen compressor is a device that increases the pressure of hydrogen by reducing its volume resulting in compressed hydrogen or liquid hydrogen.
Traditionally, applications for hydrogen compressors included Chlorine electrolyser and many chem ...
is eliminated. The average energy consumption is around 3%.
High-temperature
High-temperature electrolysis (also HTE or steam electrolysis) is more efficient at higher temperatures. Aheat engine
In thermodynamics and engineering, a heat engine is a system that converts heat to mechanical energy, which can then be used to do mechanical work. It does this by bringing a working substance from a higher state temperature to a lower state ...
supplies some of the energy, which is typically cheaper than electricity
Polymer electrolyte membrane
A proton-exchange membrane electrolyser separates reactants and transports protons while blocking a direct electronic pathway through the membrane. PEM fuel cells use a solid polymer membrane (a thin plastic film) which is permeable to protons when it is saturated with water, but it does not conduct electrons. It uses a proton-exchange membrane, or polymer-electrolyte membrane (PEM), which is asemipermeable membrane
Semipermeable membrane is a type of biological or synthetic, polymeric membrane that will allow certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecul ...
generally made from ionomer
An ionomer () ('' iono-'' + ''-mer'') is a polymer composed of repeat units of both electrically neutral repeating units and ionized units covalently bonded to the polymer backbone as pendant group moieties. Usually no more than 15 mole percent ...
s and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
and hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
gas. PEM fuel cells use a solid polymer membrane (a thin plastic film) that is permeable to protons when saturated with water, but does not conduct electrons. Proton-exchange membranes are primarily characterized by proton conductivity
Conductivity may refer to:
*Electrical conductivity, a measure of a material's ability to conduct an electric current
**Conductivity (electrolytic), the electrical conductivity of an electrolyte in solution
**Ionic conductivity (solid state), elec ...
(σ), methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
permeability (''P''), and thermal stability.
PEMs can be made from either pure polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ...
or from composite
Composite or compositing may refer to:
Materials
* Composite material, a material that is made from several different substances
** Metal matrix composite, composed of metal and other parts
** Cermet, a composite of ceramic and metallic materials
...
membranes, where other materials are embedded in a polymer matrix. One of the most common commercially available materials is the fluoropolymer
A fluoropolymer is a fluorocarbon-based polymer with multiple carbon–fluorine bonds. It is characterized by a high resistance to solvents, acids, and bases. The best known fluoropolymer is polytetrafluoroethylene under the brand name "Teflon ...
(PFSA) Nafion
Nafion is a brand name for a sulfonated tetrafluoroethylene based fluoropolymer-copolymer discovered in the late 1960s by Dr. Walther Grot of DuPont. Nafion is a brand of the Chemours company. It is the first of a class of synthetic polymers with ...
. Nafion is an ionomer with a perfluorinated backbone like Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene that has numerous applications. It is one of the best-known and widely applied PFAS. The commonly known brand name of PTFE-based composition is Teflon by Chemou ...
. Many other structural motifs are used to make ionomers for proton-exchange membranes. Many use polyaromatic polymers, while others use partially fluorinated polymers.
Supercritical water
Supercritical water electrolysis (SWE) uses water in a supercritical state. Supercritical water requires less energy, therefore reducing costs. It operates at >375°C, which reduces thermodynamic barriers and increases kinetics, improving ionic conductivity over liquid or gaseous water, which reduces ohmic losses. Benefits include improved electrical efficiency, >221bar pressurised delivery of product gases, ability to operate at high current densities and low dependence on precious metal catalysts. As of 2021 commercial SWE equipment is not available.Nickel/iron
In 2014, researchers announced electrolysis using nickel and iron catalysts rather than precious metals. Nickel-metal/nickel-oxide structure is more active than nickel metal or nickel oxide alone. The catalyst significantly lowers the requiredvoltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to m ...
. Nickel–iron batteries are under investigation for use as combined batteries and electrolysers. Those "battolysers" could be charged and discharged like conventional batteries, and would produce hydrogen when fully charged.
Nanogap electrochemical cells
In 2017, researchers reported nanogapelectrochemical cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic o ...
s that achieved high-efficiency electrolyte-free pure water electrolysis at ambient temperature. In these cells, the two electrodes are so close to each other (smaller than Debye-length) that the mass transport rate can be higher than the electron-transfer rate, leading to two half-reaction
A half reaction (or half-cell reaction) is either the oxidation or reduction reaction component of a redox reaction. A half reaction is obtained by considering the change in oxidation states of individual substances involved in the redox reaction. ...
s coupled together and limited by the electron-transfer step. Experiments show that the electrical current density can be larger than that from 1 mol/L sodium hydroxide solution. Its "Virtual Breakdown Mechanism", is completely different from traditional electrochemical theory, due to such nanogap size effects.
Capillary fed
A capillary-fed electrolyzer cell is claimed to require only 41.5 kWh to produce 1 kg of hydrogen. The water electrolyte is isolated from the electrodes by a porous, hydrophilic separator. The water is drawn into the electrolyzer by capillary action, while the electrolyzed gases pass out on either side. It extends polymer electrolyte membrane technology by eliminating bubbles that reduce the contact between the electrodes and the electrolyte, reducing efficiency. The design is claimed to operate at 98% energy efficiency. The design forgoes water circulation, separator tanks, and other mechanism and can be air- or radiatively cooled.Applications
About five percent of hydrogen gas produced worldwide is created by electrolysis. Currently, most industrial methods produce hydrogen fromnatural gas
Natural gas (also called fossil gas or simply gas) is a naturally occurring mixture of gaseous hydrocarbons consisting primarily of methane in addition to various smaller amounts of other higher alkanes. Low levels of trace gases like carbo ...
instead, in the steam reforming
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is hydrogen product ...
process. The majority of the hydrogen produced through electrolysis is a side product in the production of chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
and caustic soda
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alka ...
. This is a prime example of a competing for side reaction.
:2NaCl + 2H2O → Cl2 + H2 + 2NaOH
In the chloralkali process
The chloralkali process (also chlor-alkali and chlor alkali) is an industrial process for the electrolysis of sodium chloride (NaCl) solutions. It is the technology used to produce chlorine and sodium hydroxide (caustic soda), which are comm ...
(electrolysis of brine) a water/sodium chloride mixture is only half the electrolysis of water since the chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
ions are oxidized to chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
rather than water being oxidized to oxygen. Thermodynamically, this would not be expected since the oxidation potential of the chloride ion is less than that of water, but the rate of the chloride reaction is much greater than that of water, causing it to predominate. The hydrogen produced from this process is either burned (converting it back to water), used for the production of specialty chemicals
Speciality chemicals (also called specialties or effect chemicals) are particular chemical products which provide a wide variety of effects on which many other industry sectors rely. Some of the categories of speciality chemicals are adhesives, agr ...
, or various other small-scale applications.
Water electrolysis is also used to generate oxygen for the International Space Station
The International Space Station (ISS) is the largest modular space station currently in low Earth orbit. It is a multinational collaborative project involving five participating space agencies: NASA (United States), Roscosmos (Russia), JAXA ...
.
Additionally, many car companies have recently begun researching using water as a fuel source, converting it into hydrogen and oxygen via water electrolysis, and using the hydrogen as fuel in a Hydrogen vehicle
A hydrogen vehicle is a vehicle that uses hydrogen fuel for motive power. Hydrogen vehicles include hydrogen-fueled space rockets, as well as ships and aircraft. Power is generated by converting the chemical energy of hydrogen to mechanical energ ...
, however, have not met much success due to the unstable characteristics of hydrogen as a fuel source.
Many industrial electrolysis cells are similar to Hofmann voltameter
A Hofmann voltameter is an apparatus for electrolysing water, invented by August Wilhelm von Hofmann (1818–1892) in 1866. It consists of three joined upright cylinders, usually glass. The inner cylinder is open at the top to allow addition of ...
s, with platinum plates or honeycombs as electrodes. Generally, hydrogen is produced for point of use applications such as oxyhydrogen
Oxyhydrogen is a mixture of hydrogen (H2) and oxygen (O2) gases. This gaseous mixture is used for torches to process refractory materials and was the first
gaseous mixture used for welding. Theoretically, a ratio of 2:1 hydrogen:oxygen is enough ...
torches or when high purity hydrogen or oxygen is desired. The vast majority of hydrogen produced from hydrocarbons and as a result, contains trace amounts of carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
among other impurities. The carbon monoxide impurity can be detrimental to various systems including many fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most batteries in requ ...
s.Efficiency
Industrial output
 Efficiency of modern hydrogen generators is measured by ''energy consumed per standard volume of hydrogen'' (MJ/m3), assuming
Efficiency of modern hydrogen generators is measured by ''energy consumed per standard volume of hydrogen'' (MJ/m3), assuming standard temperature and pressure
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union o ...
of the H2. The lower the energy used by a generator, the higher its efficiency would be; a 100%-efficient electrolyser would consume (higher heating value) of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
, (page 10Archived
10 March 2016 at the
Wayback Machine
The Wayback Machine is a digital archive of the World Wide Web founded by the Internet Archive, a nonprofit based in San Francisco, California. Created in 1996 and launched to the public in 2001, it allows the user to go "back in time" and see ...
. . Practical electrolysis (using a rotating electrolyser at 15 bar pressure) may consume , and a further if the hydrogen is compressed for use in hydrogen cars. By adding external heat at , electricity consumption may be reduced.
Electrolyzer vendors provide efficiencies based on enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
. To assess the claimed efficiency of an electrolyzer it is important to establish how it was defined by the vendor (i.e. what enthalpy value, what current density, etc.).
There are three main technologies available on the market: alkaline, solid oxide, and proton exchange membrane
A proton-exchange membrane, or polymer-electrolyte membrane (PEM), is a semipermeable membrane generally made from ionomers and designed to conduct protons while acting as an electronic insulator and reactant barrier, e.g. to oxygen and hydrogen ...
(PEM) electrolyzers.
Alkaline electrolyzers are cheaper in terms of investment (they generally use nickel catalysts), but least efficient. PEM electrolyzers are more expensive (they generally use expensive platinum-group metal catalysts) but are more efficient and can operate at higher current densities, and can, therefore, be possibly cheaper if the hydrogen production is large enough. Solid oxide electrolyzer cells (SOEC) are the third most common type of electrolysis, and use high operating temperatures to increase efficiency. The theoretical electrical efficiency of SOEC is close to 100% at 90% hydrogen production. Degradation of the system over time does not affect the efficiency of SOEC electrolyzers initially unlike PEM and alkaline electrolyzers. As the SOEC system degrades, the cell voltage increases, producing more heat in the system naturally. Due to this, less energy is required to keep the system hot, which will make up for the energy losses from dramatic degradation initially.
Conventional alkaline electrolysis has an efficiency of about 70%. Accounting for the accepted use of the higher heating value
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it.
The ''calorific value'' is the total energy relea ...
(because inefficiency via heat can be redirected back into the system to create the steam required by the catalyst), average working efficiencies for PEM electrolysis Polymer electrolyte membrane (PEM) electrolysis is the electrolysis of water in a cell equipped with a solid polymer electrolyte (SPE) that is responsible for the conduction of protons, separation of product gases, and electrical insulation of the e ...
are around 80%. This is expected to increase to between 82–86% before 2030. Theoretical efficiency for PEM electrolysers are predicted up to 94%.
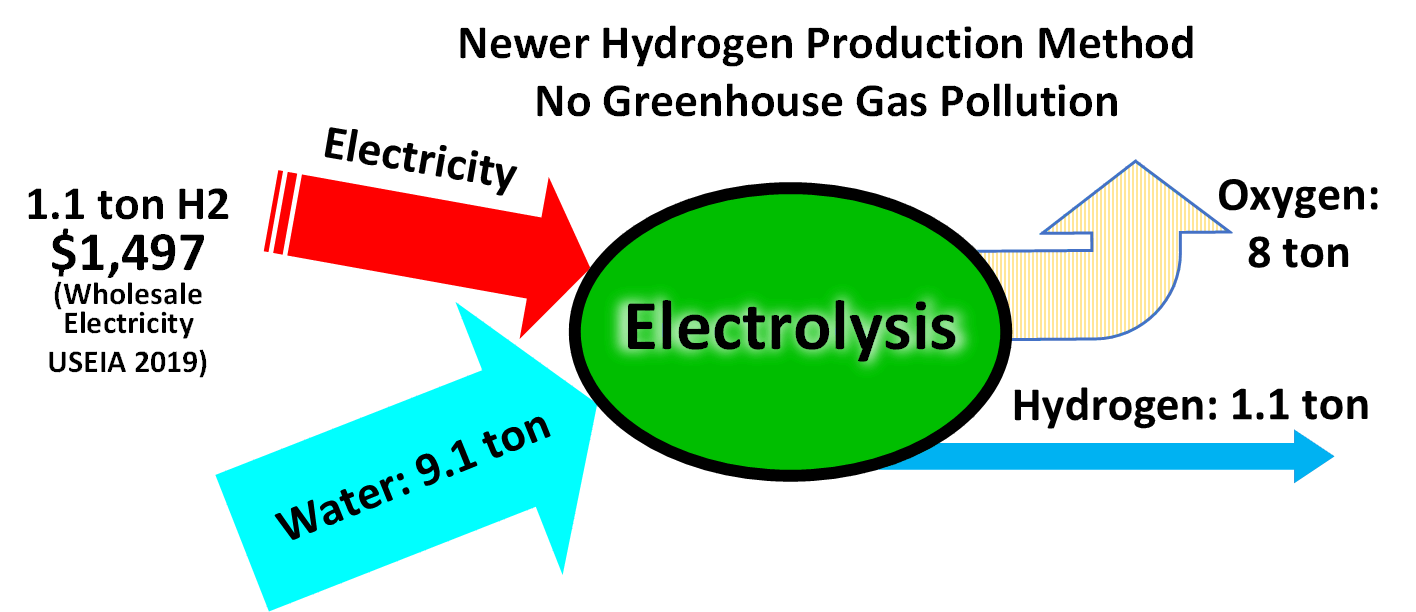
 Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–80%, producing 1 kg of hydrogen (which has a
Considering the industrial production of hydrogen, and using current best processes for water electrolysis (PEM or alkaline electrolysis) which have an effective electrical efficiency of 70–80%, producing 1 kg of hydrogen (which has a specific energy
Specific energy or massic energy is energy per unit mass. It is also sometimes called gravimetric energy density, which is not to be confused with energy density, which is defined as energy per unit volume. It is used to quantify, for example, sto ...
of 143 MJ/kg) requires of electricity. At an electricity cost of $0.06/kW·h, as set out in the US Department of Energy hydrogen production targets for 2015, the hydrogen cost is $3/kg. Equipment cost depends on mass production. , different analysts predict annual manufacture of equipment by 2030 as 47 GW, 104 GW and 180 GW, respectively.
With the range of natural gas prices from 2016 as shown in the graphHydrogen Production Tech Team Roadmap, November 2017
putting the cost of steam-methane-reformed (SMR) hydrogen at between $1.20 and $1.50, the cost price of hydrogen via electrolysis is still over double 2015 DOE hydrogen target prices. The US DOE target price for hydrogen in 2020 is $2.30/kg, requiring an electricity cost of $0.037/kW·h, which is achievable given 2018 PPA tenders for wind and solar in many regions. This puts the $4/gasoline gallon equivalent (gge) H2 dispensed objective well within reach, and close to a slightly elevated natural gas production cost for SMR. In other parts of the world, the price of SMR hydrogen is between $1–3/kg on average. This makes production of hydrogen via electrolysis cost competitive in many regions already, as outlined by Nel Hydrogen and others, including an article by the IEA examining the conditions which could lead to a competitive advantage for electrolysis. The large price increase of gas during the 2021–2022 global energy crisis made hydrogen electrolysis economic in some parts of the world. Some large industrial electrolyzers are operating at several megawatts. , the largest is a 150 MW alkaline facility in Ningxia, China, with a capacity up to 23,000 tonnes per year. While higher-efficiency Western electrolysis equipment can cost $1,200/kW, lower-efficiency Chinese equipment can cost $300/kW, but with a lower lifetime of 60,000 hours.
Overpotential
Real water electrolyzers require higher voltages for the reaction to proceed. The part that exceeds 1.23 V is calledoverpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly relat ...
or overvoltage, and represents any kind of loss and nonideality in the electrochemical process.
For a well designed cell the largest overpotential
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly relat ...
is the reaction overpotential for the four-electron oxidation of water to oxygen at the anode; electrocatalysts can facilitate this reaction, and platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Platinu ...
alloys are the state of the art for this oxidation. Developing a cheap, effective electrocatalyst for this reaction would be a great advance, and is a topic of current research; there are many approaches, among them a 30-year-old recipe for molybdenum sulfide
Molybdenum disulfide (or moly) is an inorganic compound composed of molybdenum and sulfur. Its chemical formula is .
The compound is classified as a transition metal dichalcogenide. It is a silvery black solid that occurs as the mineral molybdenit ...
, graphene quantum dots, carbon nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube
Rotating single-walled zigzag carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers.
''Single-wall carbon na ...
s, perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as (XIIA2+VIB4+X2−3), known as ...
, and nickel/nickel-oxide. Tri‐molybdenum phosphide (Mo3P) has been recently found as a promising nonprecious metal and earth‐abundant candidate with outstanding catalytic properties that can be used for electrocatalytic processes. The catalytic performance of Mo3P nanoparticles is tested in the hydrogen evolution reaction (HER), indicating an onset potential of as low as 21 mV, H2 formation rate, and exchange current density of 214.7 µmol s−1 g−1 cat (at only 100 mV overpotential) and 279.07 µA cm−2, respectively, which are among the closest values yet observed to platinum. The simpler two-electron reaction to produce hydrogen at the cathode can be electrocatalyzed with almost no overpotential by platinum, or in theory a hydrogenase enzyme. If other, less effective, materials are used for the cathode (e.g. graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large ...
), large overpotentials will appear.
Thermodynamics
The electrolysis of water in standard conditions requires a theoretical minimum of 237 kJ of electrical energy input to dissociate each mole of water, which is the standardGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pr ...
of formation of water. It also requires energy to overcome the change in entropy of the reaction. Therefore, the process cannot proceed below 286 kJ per mol if no external heat/energy is added.
Since each mole of water requires two moles of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s, and given that the Faraday constant
In physical chemistry, the Faraday constant, denoted by the symbol and sometimes stylized as ℱ, is the electric charge per mole of elementary charges. It is named after the English scientist Michael Faraday. Since the 2019 redefinition of S ...
''F'' represents the charge of a mole of electrons (96485 C/mol), it follows that the minimum voltage necessary for electrolysis is about 1.23 V. If electrolysis is carried out at high temperature, this voltage reduces. This effectively allows the electrolyser to operate at more than 100% electrical efficiency. In electrochemical systems this means that heat must be supplied to the reactor to sustain the reaction. In this way thermal energy can be used for part of the electrolysis energy requirement. In a similar way the required voltage can be reduced (below 1 V) if fuels (such as carbon, alcohol, biomass) are reacted with water (PEM based electrolyzer in low temperature) or oxygen ions (solid oxide electrolyte based electrolyzer in high temperature). This results in some of the fuel's energy being used to "assist" the electrolysis process and can reduce the overall cost of hydrogen produced.
However, observing the entropy component (and other losses), voltages over 1.48 V are required for the reaction to proceed at practical current densities (the thermoneutral voltage In electrochemistry, a thermoneutral voltage is a voltage drop across an electrochemical cell which is sufficient not only to drive the cell reaction, but to also provide the heat necessary to maintain a constant temperature. For a reaction of the f ...
).
In the case of water electrolysis, Gibbs free energy represents the minimum ''work'' necessary for the reaction to proceed, and the reaction enthalpy is the amount of energy (both work and heat) that has to be provided so the reaction products are at the same temperature as the reactant (i.e. standard temperature for the values given above). Potentially, an electrolyzer operating at 1.48 V would operate isothermally at a temperature of 25 C as the electrical energy supplied would be equal to the enthalpy (heat) of water decomposition and this would require 20% more electrical energy than the minimum.
See also
*Electrocatalyst
An electrocatalyst is a catalyst that participates in electrochemical reactions. Electrocatalysts are a specific form of catalysts that function at electrode surfaces or, most commonly, may be the electrode surface itself. An electrocatalyst ...
*Electrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference, as a measurable and quantitative phenomenon, and identifiable chemical change, with the potential difference as an outco ...
*Electrochemical cell
An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or using electrical energy to cause chemical reactions. The electrochemical cells which generate an electric current are called voltaic o ...
*Electrochemical engineering Electrochemical engineering is the branch of chemical engineering dealing with the technological applications of electrochemical phenomena, such as electrosynthesis of chemicals, electrowinning and refining of metals, flow batteries and fuel cell ...
*Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
*Gas cracker A gas cracker is any device that splits the molecules in a gas or liquid, usually by electrolysis, into atoms. The end product is usually a gas. A hydrocracker is an example of a gas cracker. In nature, molecules are split often, such as in food d ...
*Hydrogen production
Hydrogen production is the family of industrial methods for generating hydrogen gas. As of 2020, the majority of hydrogen (∼95%) is produced from fossil fuels by steam reforming of natural gas and other light hydrocarbons, partial oxidation of h ...
*Methane pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyr ...
(for Hydrogen)
*Noryl
The NORYL family of modified resins consists of amorphous blends of polyphenylene oxides (PPO) or polyphenylene ether (PPE) resins with polystyrene. They combine the inherent benefits of PPE resin (affordable high heat resistance, good electrical ...
*Photoelectrolysis of water
Photoelectrolysis of water, also known as photoelectrochemical water splitting, occurs in a photoelectrochemical cell when light is used as the energy source for the electrolysis of water, producing dihydrogen which can be used as a fuel. This pr ...
*Photocatalytic water splitting
Photocatalytic water splitting is an artificial photosynthesis process using photocatalysis for the dissociation of water (H2O) into hydrogen () and oxygen (). Only light energy (photons), water, and a catalyst(s) are needed, since this is what ...
*Electrochemical reduction of carbon dioxide
The electrochemical reduction of carbon dioxide, also known as electrolysis of carbon dioxide, is the conversion of carbon dioxide () to more reduction (chemistry), reduced chemical species using electrical energy. It is one possible step in the b ...
*Timeline of hydrogen technologies
This is a timeline of the history of hydrogen technology.
Timeline
16th century
* c. 1520 – First recorded observation of hydrogen by Paracelsus through dissolution of metals (iron, zinc, and tin) in sulfuric acid.
17th century
* 1625 – Fi ...
*Water purification
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for hu ...
References
External links
* *EERE 2008 – 100 kgH2/day Trade Study
{{DEFAULTSORT:Electrolysis Of Water Electrolysis Hydrogen production Industrial gases Water chemistry