Hydrogen Donor on:
[Wikipedia]
[Google]
[Amazon]
In
RR'C=O + Me2CHOH -> RR'C^H-OH + Me2C=O
where is a chiral product. A typical catalyst is , where Ts refers to a 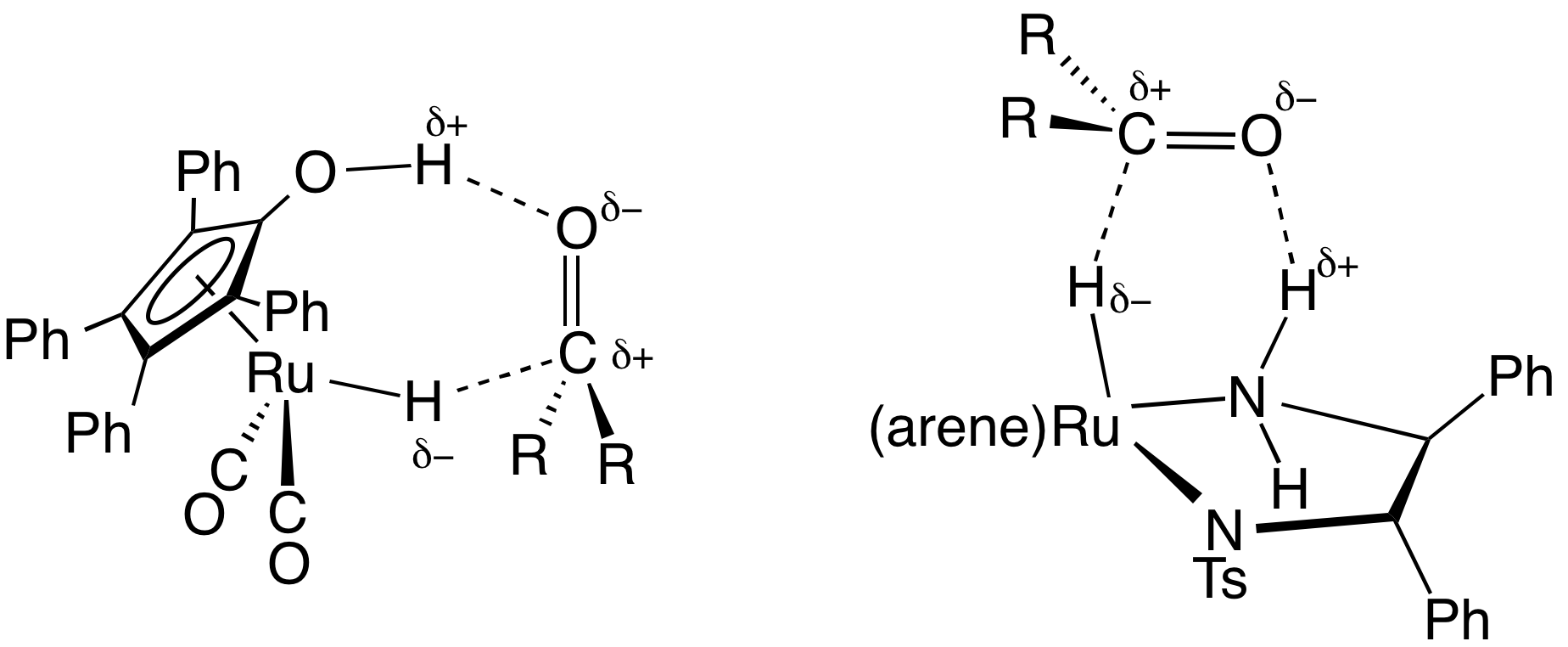
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, transfer hydrogenation is a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
involving the addition of hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
to a compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struct ...
from a source other than molecular
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
. It is applied in laboratory and industrial organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
to saturate
Saturate may refer to:
* ''Saturate'' (Breaking Benjamin album), 2002
* ''Saturate'' (Gojira album), 1999
* ''Saturate'' (Jeff Deyo album), 2002
* " Electronic Battle Weapon 8", a song by The Chemical Brothers, a shorter version of which was re ...
organic compounds and reduce
Reduction, reduced, or reduce may refer to:
Science and technology Chemistry
* Reduction (chemistry), part of a reduction-oxidation (redox) reaction in which atoms have their oxidation state changed.
** Organic redox reaction, a redox react ...
ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s to alcohols
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is ...
, and imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s to amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s. It avoids the need for high-pressure molecular used in conventional hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
catalysts, many of which are chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
, allowing efficient asymmetric synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
. It uses hydrogen donor compounds such as formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid, and has the chemical formula HCOOH and structure . It is an important intermediate in chemical synthesis and occurs naturally, most notably in some ants. Es ...
, isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
or dihydroanthracene
9,10-Dihydroanthracene is an organic compound that is derived from the polycyclic aromatic hydrocarbon anthracene. Several isomers of dihydroanthracene are known, but the 9,10 derivative is most common. It is a colourless solid that is used as a ...
, dehydrogenating them to , acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
, or anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the Economic production, production of the red dye alizarin and other dyes ...
respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction
Coal liquefaction is a process of converting coal into liquid hydrocarbons: liquid fuels and petrochemicals. This process is often known as "Coal to X" or "Carbon to X", where X can be many different hydrocarbon-based products. However, the most c ...
using "donor solvents" such as tetralin
Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. It is a partially hydrogenated derivative of naphthalene. It is a colorless liquid that is used as a hydrogen-donor solvent.
Production
Tetralin is pro ...
.
Organometallic catalysts
In the area oforganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
, a useful family of hydrogen-transfer catalysts have been developed based on ruthenium
Ruthenium is a chemical element with the Symbol (chemistry), symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to ...
and rhodium
Rhodium is a chemical element with the symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isoto ...
complexes, often with diamine
A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive.
In terms of quantities p ...
and phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
ligands. A representative catalyst precursor is derived from (cymene)ruthenium dichloride dimer and the tosylated diphenylethylenediamine
1,2-Diphenyl-1,2-ethylenediamine, DPEN, is an organic compound with the formula H2NCHPhCHPhNH2, where Ph is phenyl (C6H5). DPEN exists as three stereoisomers: meso and two enantiomers S,S- and R,R-. The chiral diastereomers are used in asymmetric ...
. These catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
are mainly employed for the reduction of ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s and imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s to alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s and amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s, respectively. The hydrogen-donor (transfer agent) is typically isopropanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simple ...
, which converts to acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscib ...
upon donation of hydrogen. Transfer hydrogenations can proceed with high enantioselectivities when the starting material is prochiral
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral.
If two identical substituents are attach ...
:
:tosyl group
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on s ...
() and ''R,R'' refers to the absolute configuration
Absolute configuration refers to the spatial arrangement of atoms within a chiral molecular entity (or group) and its resultant stereochemical description. Absolute configuration is typically relevant in organic molecules, where carbon is bonded ...
of the two chiral carbon centers. This work was recognized with the 2001 Nobel Prize in Chemistry to Ryōji Noyori
is a Japanese chemist. He won the Nobel Prize in Chemistry in 2001, Noyori shared a half of the prize with William S. Knowles for the study of chirally catalyzed hydrogenations; the second half of the prize went to K. Barry Sharpless for his ...
.
Another family of hydrogen-transfer agents are those based on aluminium alkoxides, such as aluminium isopropoxide
Aluminium isopropoxide is the chemical compound usually described with the formula Al(O-''i''-Pr)3, where ''i''-Pr is the isopropyl group (–CH(CH3)2). This colourless solid is a useful reagent in organic synthesis.
Structure
A tetrameric st ...
in the MPV reduction; however their activities are relatively low by comparison with the transition metal-based systems.

Metal-free routes
Prior to the development of catalytic hydrogenation, many methods were developed for the hydrogenation of unsaturated substrates. Many of these methods are only of historical and pedagogical interest. One prominent transfer hydrogenation agent isdiimide
Diimide, also called diazene or diimine, is a compound having the formula (NH)2. It exists as two geometric isomers, ''E'' (''trans'') and ''Z'' (''cis''). The term diazene is more common for organic derivatives of diimide. Thus, azobenzene is ...
or (NH)2, also called diazene. This becomes oxidized to the very stable N2:
The diimide is generated from hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
. Two hydrocarbons that can serve as hydrogen donors are cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorless liquid with a sharp smell. It is an intermediate in various industrial processes. Cyclohexene is not very stable upon long term storage with exposure to light an ...
or cyclohexadiene Cyclohexadiene may refer to:
* 1,3-Cyclohexadiene,
* 1,4-Cyclohexadiene,
See also
* Benzene or its theoretical isomer ''1,3,5-Cyclohexatriene''
* Cyclohexene
Cyclohexene is a hydrocarbon with the formula C6H10. This cycloalkene is a colorle ...
. In this case, an alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which ...
is formed, along with a benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
. The gain of aromatic stabilization energy when the benzene is formed is the driving force of the reaction. Pd can be used as a catalyst and a temperature of 100 °C is employed. More exotic transfer hydrogenations have been reported, including this intramolecular one:
Many reactions exist with alcohol or amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s as the proton donors, and alkali metals as electron donors. Of continuing value is the sodium metal-mediated Birch reduction
The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in an amine solvent (traditionally ...
of ''arenes'' (another name for aromatic hydrocarbons
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping ...
). Less important presently is the Bouveault–Blanc reduction
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demons ...
of esters. The combination of magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ta ...
and methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
is used in alkene reductions, e.g. the synthesis of asenapine
Asenapine, sold under the brand name Saphris among others, is an atypical antipsychotic medication used to treat schizophrenia and acute mania associated with bipolar disorder.
It was chemically derived via altering the chemical structure of the ...
:
Organocatalytic transfer hydrogenation
Organocatalytic transfer hydrogenation has been described by the group of List in 2004 in a system with aHantzsch ester
Hantzsch ester refers to an organic compound with the formula HN(MeC=C(CO2Et))2CH2 where Me = methyl (CH3) and Et = ethyl (C2H5). It is a colorless solid. The compound is an example of a 1,4- dihydropyridine. It is named after Arthur Rudolf Hant ...
as hydride donor and an amine catalyst:
In this particular reaction the substrate is an α,β-unsaturated carbonyl compound
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing ...
. The proton donor is oxidized to the pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
form and resembles the biochemically relevant coenzyme NADH
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an aden ...
. In the catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
for this reaction the amine and the aldehyde first form an iminium ion
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with al ...
, then proton transfer is followed by hydrolysis of the iminium bond regenerating the catalyst. By adopting a chiral imidazolidinone MacMillan organocatalyst an enantioselectivity
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
of 81% ee was obtained:
:
:
In a case of stereoconvergence, both the E-isomer and the Z-isomer in this reaction yield the (S)-enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
.
Extending the scope of this reaction towards ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s or rather enones requires fine tuning of the catalyst (add a benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
group and replace the t-butyl
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane.
The isomer ''n''-butane can connect in two ways, givi ...
group by a furan
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans.
Furan is a colorless, flammable, highly ...
) and of the Hantzsch ester (add more bulky t-butyl groups):
:
With another organocatalyst altogether, hydrogenation can also be accomplished for imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s. One cascade reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the p ...
is catalyzed by a chiral phosphoric acid:{{Cite journal, author1 = Rueping, first2 = A., first3 = T., title = A highly enantioselective Brønsted acid catalyzed cascade reaction: organocatalytic transfer hydrogenation of quinolines and their application in the synthesis of alkaloids, journal = Angewandte Chemie International Edition in English, volume = 45, issue = 22, pages = 3683–3686, year = 2006, pmid = 16639754, doi = 10.1002/anie.200600191, last2 = Antonchick, last3 = Theissmann
:
The reaction proceeds via a chiral iminium ion
In organic chemistry, an iminium cation is a polyatomic ion with the general structure . They are common in synthetic chemistry and biology.
Structure
Iminium cations adopt alkene-like geometries. The central C=N unit is nearly coplanar with al ...
. Traditional metal-based catalysts, hydrogenation of aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
or heteroaromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturat ...
substrates tend to fail.
References
See also
*Meerwein–Ponndorf–Verley reduction
The Meerwein–Ponndorf–Verley (MPV) reduction in organic chemistry is the reduction of ketones and aldehydes to their corresponding alcohols utilizing aluminium alkoxide catalysis in the presence of a sacrificial alcohol. The advantages of the ...
* Oppenauer oxidation
Oppenauer oxidation, named after , is a gentle method for selectively oxidizing secondary alcohols to ketones.
The reaction is the opposite Meerwein–Ponndorf–Verley reduction. The alcohol is oxidized with aluminium isopropoxide in excess ace ...
* Dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
* Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ...
* Hydrogenolysis
Hydrogenolysis is a chemical reaction whereby a carbon–carbon or carbon–heteroatom single bond is cleaved or undergoes lysis (breakdown) by hydrogen.Ralph Connor, Homer Adkins. Hydrogenolysis Of Oxygenated Organic Compounds. J. Am. Chem. Soc. ...
* Borrowing hydrogen
Hydrogen auto-transfer, also known as borrowing hydrogen, is the activation of a chemical reaction by temporary transfer of two hydrogen atoms from the reactant to a catalyst and return of those hydrogen atoms back to a reaction intermediate to fo ...
Chemical processes
Industrial processes
Organic redox reactions
Hydrogenation