Humid Peroxide Oxidation on:
[Wikipedia]
[Google]
[Amazon]
Advanced oxidation processes (AOPs), in a broad sense, are a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in
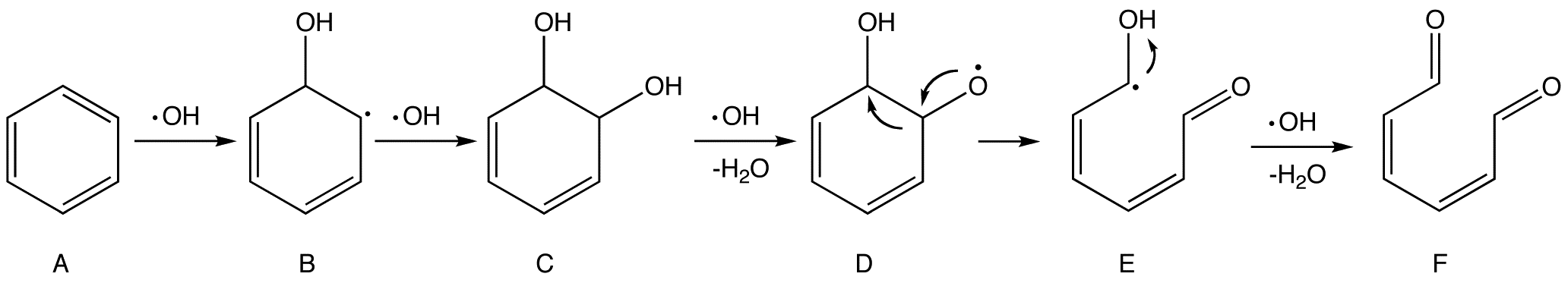 ''Scheme 1. Proposed mechanism of the oxidation of benzene by hydroxyl radicals''
The first and second steps are electrophilic addition that breaks the aromatic ring in benzene (A) and forms two hydroxyl groups (–OH) in intermediate C. Later an ·OH grabs a hydrogen atom in one of the hydroxyl groups, producing a radical species (D) that is prone to undergo rearrangement to form a more stable radical (E). E, on the other hand, is readily attacked by ·OH and eventually forms 2,4-hexadiene-1,6-dione (F).
As long as there are sufficient ·OH radicals, subsequent attacks on compound F will continue until the fragments are all converted into small and stable molecules like H2O and CO2 in the end, but such processes may still be subject to a myriad of possible and partially unknown mechanisms.
''Scheme 1. Proposed mechanism of the oxidation of benzene by hydroxyl radicals''
The first and second steps are electrophilic addition that breaks the aromatic ring in benzene (A) and forms two hydroxyl groups (–OH) in intermediate C. Later an ·OH grabs a hydrogen atom in one of the hydroxyl groups, producing a radical species (D) that is prone to undergo rearrangement to form a more stable radical (E). E, on the other hand, is readily attacked by ·OH and eventually forms 2,4-hexadiene-1,6-dione (F).
As long as there are sufficient ·OH radicals, subsequent attacks on compound F will continue until the fragments are all converted into small and stable molecules like H2O and CO2 in the end, but such processes may still be subject to a myriad of possible and partially unknown mechanisms.
water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
and wastewater
Wastewater is water generated after the use of freshwater, raw water, drinking water or saline water in a variety of deliberate applications or processes. Another definition of wastewater is "Used water from any combination of domestic, industr ...
by oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
through reactions with hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
s (·OH). In real-world applications of wastewater treatment
Wastewater treatment is a process used to remove contaminants from wastewater and convert it into an effluent that can be returned to the water cycle. Once returned to the water cycle, the effluent creates an acceptable impact on the environme ...
, however, this term usually refers more specifically to a subset of such chemical processes that employ ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
(O3), hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
(H2O2) and/or UV light. One such type of process is called in situ chemical oxidation
In situ chemical oxidation (ISCO), a form of advanced oxidation process, is an environmental remediation technique used for soil and/or groundwater remediation to lower the concentrations of targeted environmental contaminants to acceptable leve ...
.
Description
AOPs rely on in-situ production of highly reactive hydroxyl radicals (·OH). These reactive species are the strongest oxidants that can be applied in water and can oxidize virtually any compound present in the water matrix, often at a diffusion-controlled reaction speed. Consequently, ·OH reacts unselectively once formed and contaminants will be quickly and efficiently fragmented and converted into small inorganic molecules. Hydroxyl radicals are produced with the help of one or more primary oxidants (e.g.ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
, hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
) and/or energy sources (e.g. ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nanometer, nm (with a corresponding frequency around 30 Hertz, PHz) to 400 nm (750 Hertz, THz), shorter than that of visible light, but longer than ...
light) or catalysts (e.g. titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insolubl ...
). Precise, pre-programmed dosages, sequences and combinations of these reagents are applied in order to obtain a maximum •OH yield. In general, when applied in properly tuned conditions, AOPs can reduce the concentration of contaminants from several-hundreds ppm to less than 5 ppb and therefore significantly bring COD
Cod is the common name for the demersal fish genus '' Gadus'', belonging to the family Gadidae. Cod is also used as part of the common name for a number of other fish species, and one species that belongs to genus ''Gadus'' is commonly not call ...
and TOC down, which earned it the credit of “water treatment processes of the 21st century”.
The AOP procedure is particularly useful for cleaning biologically toxic or non-degradable materials such as aromatics
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping ...
, pesticides
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampric ...
, petroleum
Petroleum, also known as crude oil, or simply oil, is a naturally occurring yellowish-black liquid mixture of mainly hydrocarbons, and is found in geological formations. The name ''petroleum'' covers both naturally occurring unprocessed crud ...
constituents, and volatile organic compound
Volatile organic compounds (VOCs) are organic compounds that have a high vapour pressure at room temperature. High vapor pressure correlates with a low boiling point, which relates to the number of the sample's molecules in the surrounding air, a ...
s in wastewater. Additionally, AOPs can be used to treat effluent of secondary treated wastewater which is then called tertiary treatment
Sewage treatment (or domestic wastewater treatment, municipal wastewater treatment) is a type of wastewater treatment which aims to remove contaminants from sewage to produce an effluent that is suitable for discharge to the surrounding envir ...
. The contaminant materials are largely converted into stable inorganic compounds such as water, carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
and salts, i.e. they undergo mineralization
Mineralization may refer to:
* Mineralization (biology), when an inorganic substance precipitates in an organic matrix
** Biomineralization, a form of mineralization
** Mineralization of bone, an example of mineralization
** Mineralized tissues are ...
. A goal of the wastewater purification by means of AOP procedures is the reduction of the chemical contaminants and the toxicity to such an extent that the cleaned wastewater may be reintroduced into receiving streams or, at least, into a conventional sewage treatment
Sewage treatment (or domestic wastewater treatment, municipal wastewater treatment) is a type of wastewater treatment which aims to remove contaminants from sewage to produce an effluent that is suitable for discharge to the surrounding envir ...
.
Although oxidation processes involving ·OH have been in use since late 19th century (such as Fenton's reagent
Fenton's reagent is a solution of hydrogen peroxide (H2O2) with ferrous iron (typically iron(II) sulfate, FeSO4) as a catalyst that is used to oxidize contaminants or waste waters as part of an advanced oxidation process. Fenton's reagent can be us ...
, which was used as an analytical reagent at that time), the utilization of such oxidative species in water treatment did not receive adequate attention until Glaze et al. suggested the possible generation of ·OH “in sufficient quantity to affect water purification” and defined the term “Advanced Oxidation Processes” for the first time in 1987. AOPs still have not been put into commercial use on a large scale (especially in developing countries) even up to today mostly because of relatively high associated costs. Nevertheless, its high oxidative capability and efficiency make AOPs a popular technique in tertiary treatment in which the most recalcitrant organic and inorganic contaminants are to be eliminated. The increasing interest in water reuse
Water reclamation (also called wastewater reuse, water reuse or water recycling) is the process of converting municipal wastewater (sewage) or industrial wastewater into water that can be reused for a variety of purposes. Types of reuse include: ...
and more stringent regulations regarding water pollution are currently accelerating the implementation of AOPs at full-scale.
There are roughly 500 commercialized AOP installations around the world at present, mostly in Europe
Europe is a large peninsula conventionally considered a continent in its own right because of its great physical size and the weight of its history and traditions. Europe is also considered a Continent#Subcontinents, subcontinent of Eurasia ...
and the United States
The United States of America (U.S.A. or USA), commonly known as the United States (U.S. or US) or America, is a country primarily located in North America. It consists of 50 states, a federal district, five major unincorporated territorie ...
. Other countries like China
China, officially the People's Republic of China (PRC), is a country in East Asia. It is the world's most populous country, with a population exceeding 1.4 billion, slightly ahead of India. China spans the equivalent of five time zones and ...
are showing increasing interests in AOPs.
Chemical principles
Generally speaking, chemistry in AOPs could be essentially divided into three parts: # Formation of ·OH; # Initial attacks on target molecules by ·OH and their breakdown to fragments; # Subsequent attacks by ·OH until ultimatemineralization
Mineralization may refer to:
* Mineralization (biology), when an inorganic substance precipitates in an organic matrix
** Biomineralization, a form of mineralization
** Mineralization of bone, an example of mineralization
** Mineralized tissues are ...
.
The mechanism of ·OH production (Part 1) highly depends on the sort of AOP technique that is used. For example, ozonation, UV/H2O2, photocatalytic oxidation and Fenton's oxidation rely on different mechanisms of ·OH generation:
*UV/H2O2:
:H2O2 + UV → 2·OH ''(homolytic bond cleavage of the O-O bond of H2O2 leads to formation of 2·OH radicals)''
* UV/HOCl:
:HOCl + UV → ·OH + Cl·
*Ozone based AOP:
:O3 + HO− → HO2− + O2 ''(reaction between O3 and a hydroxyl ion leads to the formation of H2O2 (in charged form))''
:O3 + HO2− → HO2· + O3−· ''(a second O3 molecule reacts with the HO2− to produce the ozonide radical)''
:O3−· + H+ → HO3· ''(this radical gives to ·OH upon protonation)''
:HO3· → ·OH + O2
:''the reaction steps presented here are just a part of the reaction sequence, see reference for more details''
* Fenton based AOP:
Fe2+ + H2O2 → Fe3++ HO· + OH− (initiation of Fenton's reagent)
Fe3+ + H2O2 → Fe2++ HOO· + H+ (regeneration of Fe2+ catalyst)
H2O2 → HO· + HOO· + H2O (Self scavenging and decomposition of H2O2)
''the reaction steps presented here are just a part of the reaction sequence, see reference for more details''
*Photocatalytic oxidation with TiO2:
:TiO2 + UV → e− + h+ ''(irradiation of the photocatalytic surface leads to an excited electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
(e−) and electron gap (h+))''
:Ti(IV) + H2O Ti(IV)-H2O ''(water adsorbs onto the catalyst surface)''
:Ti(IV)-H2O + h+ Ti(IV)-·OH + H+ ''the highly reactive electron gap will react with water''
:''the reaction steps presented here are just a part of the reaction sequence, see reference for more details''
Currently there is no consensus on the detailed mechanisms in Part 3, but researchers have cast light on the processes of initial attacks in Part 2. In essence, ·OH is a radical species and should behave like a highly reactive electrophile. Thus two type of initial attacks are supposed to be Hydrogen Abstraction
In chemistry, a hydrogen atom abstraction or hydrogen atom transfer (HAT) is any chemical reaction in which a hydrogen free radical (neutral hydrogen atom) is abstracted from a substrate according to the general equation:
:X^\bullet + H-Y -> X-H ...
and Addition
Addition (usually signified by the Plus and minus signs#Plus sign, plus symbol ) is one of the four basic Operation (mathematics), operations of arithmetic, the other three being subtraction, multiplication and Division (mathematics), division. ...
. The following scheme, adopted from a technical handbook and later refined, describes a possible mechanism of the oxidation of benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
by ·OH.
 ''Scheme 1. Proposed mechanism of the oxidation of benzene by hydroxyl radicals''
The first and second steps are electrophilic addition that breaks the aromatic ring in benzene (A) and forms two hydroxyl groups (–OH) in intermediate C. Later an ·OH grabs a hydrogen atom in one of the hydroxyl groups, producing a radical species (D) that is prone to undergo rearrangement to form a more stable radical (E). E, on the other hand, is readily attacked by ·OH and eventually forms 2,4-hexadiene-1,6-dione (F).
As long as there are sufficient ·OH radicals, subsequent attacks on compound F will continue until the fragments are all converted into small and stable molecules like H2O and CO2 in the end, but such processes may still be subject to a myriad of possible and partially unknown mechanisms.
''Scheme 1. Proposed mechanism of the oxidation of benzene by hydroxyl radicals''
The first and second steps are electrophilic addition that breaks the aromatic ring in benzene (A) and forms two hydroxyl groups (–OH) in intermediate C. Later an ·OH grabs a hydrogen atom in one of the hydroxyl groups, producing a radical species (D) that is prone to undergo rearrangement to form a more stable radical (E). E, on the other hand, is readily attacked by ·OH and eventually forms 2,4-hexadiene-1,6-dione (F).
As long as there are sufficient ·OH radicals, subsequent attacks on compound F will continue until the fragments are all converted into small and stable molecules like H2O and CO2 in the end, but such processes may still be subject to a myriad of possible and partially unknown mechanisms.
Advantages
AOPs hold several advantages in the field of water treatment: *They can effectively eliminate organic compounds in aqueous phase, rather than collecting or transferring pollutants into another phase. *Due to the reactivity of ·OH, it reacts with many aqueous pollutants without discriminating. AOPs are therefore applicable in many, if not all, scenarios where many organic contaminants must be removed at the same time. *Someheavy metals
upright=1.2, Crystals of osmium, a heavy metal nearly twice as dense as lead">lead.html" ;"title="osmium, a heavy metal nearly twice as dense as lead">osmium, a heavy metal nearly twice as dense as lead
Heavy metals are generally defined as ...
can also be removed in forms of precipitated M(OH)x.
*In some AOPs designs, disinfection
A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than st ...
can also be achieved, which makes these AOPs an integrated solution to some water quality problems.
*Since the complete reduction product of ·OH is H2O, AOPs theoretically do not introduce any new hazardous substances into the water.
Current shortcomings
AOPs are not perfect and have several drawbacks. *Most prominently, the cost of AOPs is fairly high, since a continuous input of expensive chemical reagents is required to maintain the operation of most AOP systems. As a result of their very nature, AOPs require hydroxyl radicals and other reagents proportional to the quantity of contaminants to be removed. *Some techniques require pre-treatment of wastewater to ensure reliable performance, which could be potentially costly and technically demanding. For instance, presence ofbicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial biochemic ...
ions (HCO3−) can appreciably reduce the concentration of ·OH due to scavenging processes that yield H2O and a much less reactive species, ·CO3−. As a result, bicarbonate must be wiped out from the system or AOPs are compromised.
*It is not cost effective to use solely AOPs to handle a large amount of wastewater; instead, AOPs should be deployed in the final stage after primary
Primary or primaries may refer to:
Arts, entertainment, and media Music Groups and labels
* Primary (band), from Australia
* Primary (musician), hip hop musician and record producer from South Korea
* Primary Music, Israeli record label
Works
* ...
and secondary treatment
Secondary treatment is the removal of biodegradable organic matter (in solution or suspension) from sewage or similar kinds of wastewater. The aim is to achieve a certain degree of effluent quality in a sewage treatment plant suitable for the ...
have successfully removed a large proportion of contaminants. Ongoing research also been done to combine AOPs with biological treatment to bring the cost down.
Future
Since AOPs were first defined in 1987, the field has witnessed a rapid development both in theory and in application. So far, TiO2/UV systems, H2O2/UV systems, and Fenton, photo-Fenton and Electro-Fenton systems have received extensive scrutiny. However, there are still many research needs on these existing AOPs. Recent trends are the development of new, modified AOPs that are efficient and economical. In fact, there has been some studies that offer constructive solutions. For instance, doping TiO2 with non-metallic elements could possibly enhance thephotocatalytic
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a catalyst. In catalyzed photolysis, light is absorbed by an adsorbed substrate. In photogenerated catalysis, the photocatalytic activity depends on the abi ...
activity; and implementation of ultrasonic treatment could promote the production of hydroxyl radicals. Modified AOPs such as Fluidized-Bed Fenton has also shown great potential in terms of degradation performance and economics.
See also
*List of waste-water treatment technologies
This page consists of a list of wastewater treatment technologies:
See also
*Agricultural wastewater treatment
*Industrial wastewater treatment
*List of solid waste treatment technologies
* Waste treatment technologies
*Water purification
*Sewa ...
* Fenton reaction
Fenton's reagent is a solution of hydrogen peroxide (H2O2) with ferrous iron (typically iron(II) sulfate, FeSO4) as a catalyst that is used to oxidize contaminants or waste waters as part of an advanced oxidation process. Fenton's reagent can be us ...
*Electro-oxidation
Electro-oxidation (EO), also known as anodic oxidation or electrochemical oxidation, is a technique used for wastewater treatment, mainly for industrial effluents, and is a type of advanced oxidation process (AOP). The most general layout comprises ...
* Process engineering
Process engineering is the understanding and application of the fundamental principles and laws of nature that allow humans to transform raw material and energy into products that are useful to society, at an industrial level. By taking advantage ...
* Water purification
Water purification is the process of removing undesirable chemicals, biological contaminants, suspended solids, and gases from water. The goal is to produce water that is fit for specific purposes. Most water is purified and disinfected for hu ...
References
Further reading
*Michael OD Roth: ''Chemical oxidation: Technology for the Nineties, volume VI: Technologies for the Nineties: 6 (Chemical oxidation)'' W. Wesley corner fields and John A. Roth, Technomic Publishing CO, Lancaster among other things. 1997, . (engl.) * {{DEFAULTSORT:Advanced Oxidation Process Water treatment Environmental engineering Environmental chemistry Green chemistry