History Of Mass Spectrometry on:
[Wikipedia]
[Google]
[Amazon]

 The history of mass spectrometry has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode and cathode rays, which turned out to be positive ions and electrons. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotopes of the elements. The first such discovery was with the element
The history of mass spectrometry has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode and cathode rays, which turned out to be positive ions and electrons. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotopes of the elements. The first such discovery was with the element
 In the mid-nineteenth century, Julius Plücker investigated the light emitted in
In the mid-nineteenth century, Julius Plücker investigated the light emitted in
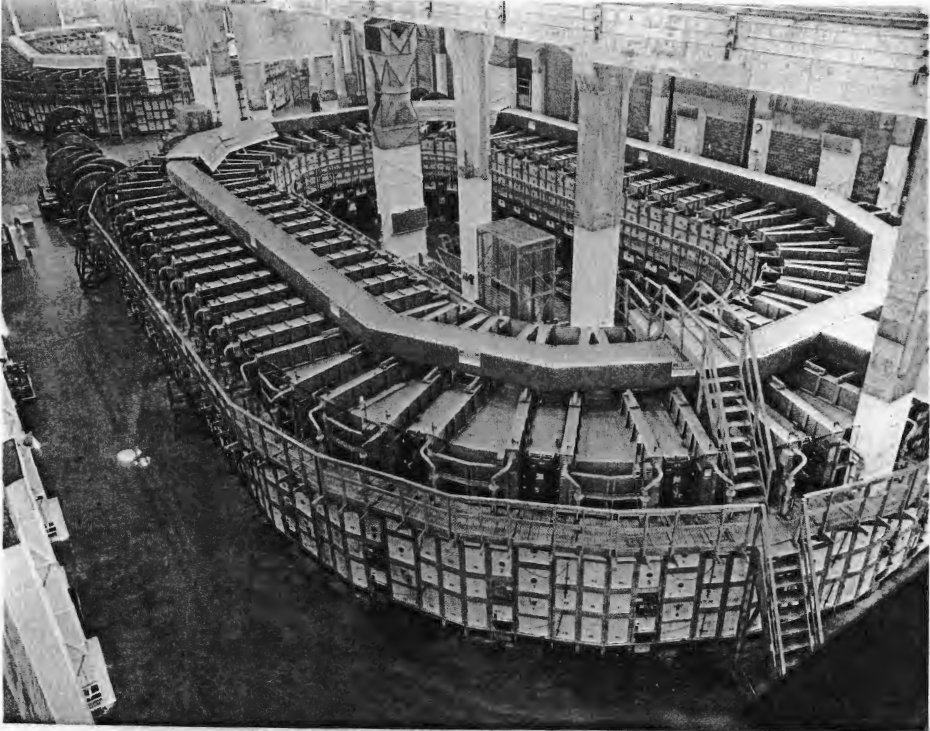 A Calutron is a sector mass spectrometer that was used for separating the isotopes of uranium developed by Ernest O. Lawrence during the Manhattan Project and was similar to the Cyclotron invented by Lawrence. Its name is a concatenation of Cal. U.-tron, in tribute to the University of California, Lawrence's institution and the contractor of the Los Alamos laboratory., url=http://masspec.scripps.edu/MSHistory/timelines/time_pdf/1947_ParkinsWE.pdf, format=PDF, accessdate=2007-09-01 They were implemented for industrial scale uranium enrichment at the
A Calutron is a sector mass spectrometer that was used for separating the isotopes of uranium developed by Ernest O. Lawrence during the Manhattan Project and was similar to the Cyclotron invented by Lawrence. Its name is a concatenation of Cal. U.-tron, in tribute to the University of California, Lawrence's institution and the contractor of the Los Alamos laboratory., url=http://masspec.scripps.edu/MSHistory/timelines/time_pdf/1947_ParkinsWE.pdf, format=PDF, accessdate=2007-09-01 They were implemented for industrial scale uranium enrichment at the
 Field desorption ionization was first reported by Beckey in 1969. In field ionization, a high-potential electric field is applied to an ''emitter'' with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have been grown. This produces a very high electric field in which electron tunneling can result in ionization of gaseous analyte molecules. FI produces mass spectra with little or no fragmentation, dominated by molecular radical cations M+. and occasionally protonated molecules .
Chemical ionization was developed in the 1960s. Ionization of sample (analyte) is achieved by interaction of its molecules with reagent ions. The analyte is ionized by ion-molecule reactions during collisions in the source. The process may involve transfer of an electron, a proton or other charged species between the reactants. This is a less energetic procedure than
Field desorption ionization was first reported by Beckey in 1969. In field ionization, a high-potential electric field is applied to an ''emitter'' with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have been grown. This produces a very high electric field in which electron tunneling can result in ionization of gaseous analyte molecules. FI produces mass spectra with little or no fragmentation, dominated by molecular radical cations M+. and occasionally protonated molecules .
Chemical ionization was developed in the 1960s. Ionization of sample (analyte) is achieved by interaction of its molecules with reagent ions. The analyte is ionized by ion-molecule reactions during collisions in the source. The process may involve transfer of an electron, a proton or other charged species between the reactants. This is a less energetic procedure than
 :: Wilhelm Wien demonstrates that canal rays can be deflected using strong electric and magnetic fields. He shows that the
:: Wilhelm Wien demonstrates that canal rays can be deflected using strong electric and magnetic fields. He shows that the
 :: Ernest O. Lawrence invents the cyclotron.
:1934
::
:: Ernest O. Lawrence invents the cyclotron.
:1934
::Internet Archive Wayback Machine
/ref>
History of Mass Spectrometry - Pioneers - University of New South Wales Sydney
* ttps://web.archive.org/web/20071108220147/http://masspec.scripps.edu/mshistory/mshistory.php History of Mass Spectrometry - Scripps Institute {{Mass spectrometry Mass spectrometry

 The history of mass spectrometry has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode and cathode rays, which turned out to be positive ions and electrons. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotopes of the elements. The first such discovery was with the element
The history of mass spectrometry has its roots in physical and chemical studies regarding the nature of matter. The study of gas discharges in the mid 19th century led to the discovery of anode and cathode rays, which turned out to be positive ions and electrons. Improved capabilities in the separation of these positive ions enabled the discovery of stable isotopes of the elements. The first such discovery was with the element neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton ...
, which was shown by mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
to have at least two stable isotopes: 20Ne (neon with 10 proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s and 10 neutrons) and 22Ne (neon with 10 protons and 12 neutrons). Mass spectrometers were used in the Manhattan Project for the separation of isotopes of uranium necessary to create the atomic bomb
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions (thermonuclear bomb), producing a nuclear explosion. Both bomb ...
.
Prout's Hypothesis
Prout's hypothesis
Prout's hypothesis was an early 19th-century attempt to explain the existence of the various chemical elements through a hypothesis regarding the internal structure of the atom. In 1815 and 1816, the English chemist William Prout published two p ...
was an early 19th-century attempt to explain the properties of the chemical elements using the internal structure of the atom. In 1815, the English chemist William Prout observed that the atomic weights that had been measured were integer multiples of the atomic weight of hydrogen. Prout's hypothesis remained influential in chemistry throughout the 1820s. However, more careful measurements of the atomic weights, such as those compiled by Jöns Jakob Berzelius in 1828 or Edward Turner in 1832, appeared to disprove it. In particular the atomic weight of chlorine, which is 35.45 times that of hydrogen, could not at the time be explained in terms of Prout's hypothesis. It would take the better part of a century for this problem to be resolved.
Canal rays
 In the mid-nineteenth century, Julius Plücker investigated the light emitted in
In the mid-nineteenth century, Julius Plücker investigated the light emitted in discharge tubes
A gas-filled tube, also commonly known as a discharge tube or formerly as a Plücker tube, is an arrangement of electrodes in a gas within an insulating, temperature-resistant envelope. Gas-filled tubes exploit phenomena related to electric d ...
and the influence of magnetic fields on the glow. Later, in 1869, Johann Wilhelm Hittorf
Johann Wilhelm Hittorf (27 March 1824 – 28 November 1914) was a German physicist who was born in Bonn and died in Münster, Germany.
Hittorf was the first to compute the electricity-carrying capacity of charged atoms and molecules (ions), an ...
studied discharge tubes with energy rays extending from a negative electrode, the cathode. These rays produced a fluorescence when they hit a tube's glass walls, and when interrupted by a solid object they cast a shadow.
Canal rays, also called anode rays
An anode ray (also positive ray or canal ray) is a beam of positive ions that is created by certain types of gas-discharge tubes. They were first observed in Crookes tubes during experiments by the German scientist Eugen Goldstein, in 1886. Later ...
, were observed by Eugen Goldstein
Eugen Goldstein (; 5 September 1850 – 25 December 1930) was a German physicist. He was an early investigator of discharge tubes, the discoverer of anode rays or canal rays, later identified as positive ions in the gas phase including the hy ...
, in 1886. Goldstein used a gas discharge tube which had a perforated cathode. The rays are produced in the holes (canals) in the cathode and travels in a direction opposite to the " cathode rays," which are streams of electrons. Goldstein called these positive rays "Kanalstrahlen" - canal rays.
Discovery of isotopes
In 1913, as part of his exploration into the composition of canal rays, J. J. Thomson channeled a stream of ionized neon through a magnetic and an electric field and measured its deflection by placing a photographic plate in its path. Thomson observed two patches of light on the photographic plate (see image on left), which suggested two different parabolas of deflection. Thomson concluded that the neon gas was composed of atoms of two different atomic masses (neon-20 and neon-22). Thomson's student Francis William Aston continued the research at the Cavendish Laboratory in Cambridge, building the first full functional mass spectrometer that was reported in 1919. He was able to identify isotopes of chlorine (35 and 37), bromine (79 and 81), and krypton (78, 80, 82, 83, 84 and 86), proving that these natural occurring elements are composed of a combination of isotopes. The use of electromagnetic focusing in mass spectrograph which rapidly allowed him to identify no fewer than 212 of the 287 naturally occurring isotopes. In 1921, F. W. Aston became a fellow of the Royal Society and received a Nobel Prize in Chemistry in the following year. His work on isotopes also led to his formulation of the Whole Number Rule which states that "the mass of the oxygen isotope being defineds 16 S16 may refer to:
Automobiles
* Chery QQme, a Chinese city car
* Peugeot 306 S16, a French family car
* Proton S16, a Malaysian subcompact car
Aviation
* Copalis State Airport, in Grays Harbor County, Washington, United States
* Letov Š-16, ...
all the other isotopes have masses that are very nearly whole numbers," a rule that was used extensively in the development of nuclear energy
Nuclear energy may refer to:
*Nuclear power, the use of sustained nuclear fission or nuclear fusion to generate heat and electricity
* Nuclear binding energy, the energy needed to fuse or split a nucleus of an atom
*Nuclear potential energy
...
. The exact mass of many isotopes was measured leading to the result that hydrogen has a 1% higher mass than expected by the average mass of the other elements. Aston speculated about the subatomic energy and the use of it in 1936.
In 1918, Arthur Jeffrey Dempster reported on his mass spectrometer and established the basic theory and design of mass spectrometers that is still used to this day. Dempster's research over his career centered around the mass spectrometer and its applications, leading in 1935 to his discovery of the uranium isotope 235U. This isotope's ability to cause a rapidly expanding fission
Fission, a splitting of something into two or more parts, may refer to:
* Fission (biology), the division of a single entity into two or more parts and the regeneration of those parts into separate entities resembling the original
* Nuclear fissio ...
nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
allowed the development of the atom bomb and nuclear power.
In 1932, Kenneth Bainbridge
Kenneth Tompkins Bainbridge (July 27, 1904 – July 14, 1996) was an American physicist at Harvard University who did work on cyclotron research. His precise measurements of mass differences between nuclear isotopes allowed him to confirm Albert ...
developed a mass spectrometer with a resolving power of 600 and a relative precision of one part in 10,000. He used this instrument to verify the equivalence of mass and energy, E = mc2.
Manhattan Project
 A Calutron is a sector mass spectrometer that was used for separating the isotopes of uranium developed by Ernest O. Lawrence during the Manhattan Project and was similar to the Cyclotron invented by Lawrence. Its name is a concatenation of Cal. U.-tron, in tribute to the University of California, Lawrence's institution and the contractor of the Los Alamos laboratory., url=http://masspec.scripps.edu/MSHistory/timelines/time_pdf/1947_ParkinsWE.pdf, format=PDF, accessdate=2007-09-01 They were implemented for industrial scale uranium enrichment at the
A Calutron is a sector mass spectrometer that was used for separating the isotopes of uranium developed by Ernest O. Lawrence during the Manhattan Project and was similar to the Cyclotron invented by Lawrence. Its name is a concatenation of Cal. U.-tron, in tribute to the University of California, Lawrence's institution and the contractor of the Los Alamos laboratory., url=http://masspec.scripps.edu/MSHistory/timelines/time_pdf/1947_ParkinsWE.pdf, format=PDF, accessdate=2007-09-01 They were implemented for industrial scale uranium enrichment at the Oak Ridge, Tennessee
Oak Ridge is a city in Anderson and Roane counties in the eastern part of the U.S. state of Tennessee, about west of downtown Knoxville. Oak Ridge's population was 31,402 at the 2020 census. It is part of the Knoxville Metropolitan Area. Oak ...
Y-12 plant
The Y-12 National Security Complex is a United States Department of Energy National Nuclear Security Administration facility located in Oak Ridge, Tennessee, near the Oak Ridge National Laboratory. It was built as part of the Manhattan Projec ...
established during the war and provided much of the uranium used for the " Little Boy" nuclear weapon, which was dropped onto Hiroshima
is the capital of Hiroshima Prefecture in Japan. , the city had an estimated population of 1,199,391. The gross domestic product (GDP) in Greater Hiroshima, Hiroshima Urban Employment Area, was US$61.3 billion as of 2010. Kazumi Matsui h ...
in 1945.
Development of gas chromatography-mass spectrometry
The use of a mass spectrometer as the detector in gas chromatography was developed during the 1950s by Roland Gohlke and Fred McLafferty. The development of affordable and miniaturized computers has helped in the simplification of the use of this instrument, as well as allowed great improvements in the amount of time it takes to analyze a sample.Fourier transform mass spectrometry
Fourier transform ion cyclotron resonance mass spectrometry was developed by Alan G. Marshall and Melvin B. Comisarow at the University of British Columbia in 1974. The inspiration was earlier developments in conventional ICR and Fourier Transform Nuclear Magnetic Resonance (FT-NMR) spectroscopy.Soft ionization methods
 Field desorption ionization was first reported by Beckey in 1969. In field ionization, a high-potential electric field is applied to an ''emitter'' with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have been grown. This produces a very high electric field in which electron tunneling can result in ionization of gaseous analyte molecules. FI produces mass spectra with little or no fragmentation, dominated by molecular radical cations M+. and occasionally protonated molecules .
Chemical ionization was developed in the 1960s. Ionization of sample (analyte) is achieved by interaction of its molecules with reagent ions. The analyte is ionized by ion-molecule reactions during collisions in the source. The process may involve transfer of an electron, a proton or other charged species between the reactants. This is a less energetic procedure than
Field desorption ionization was first reported by Beckey in 1969. In field ionization, a high-potential electric field is applied to an ''emitter'' with a sharp surface, such as a razor blade, or more commonly, a filament from which tiny "whiskers" have been grown. This produces a very high electric field in which electron tunneling can result in ionization of gaseous analyte molecules. FI produces mass spectra with little or no fragmentation, dominated by molecular radical cations M+. and occasionally protonated molecules .
Chemical ionization was developed in the 1960s. Ionization of sample (analyte) is achieved by interaction of its molecules with reagent ions. The analyte is ionized by ion-molecule reactions during collisions in the source. The process may involve transfer of an electron, a proton or other charged species between the reactants. This is a less energetic procedure than electron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of th ...
and the ions produced are, for example, protonated molecules: + Hsup>+. These ions are often relatively stable, tending not to fragment as readily as ions produced by electron ionization
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of th ...
.
Matrix-assisted laser desorption/ionization (MALDI) is a soft ionization technique used in mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
, allowing the analysis of biomolecule
A biomolecule or biological molecule is a loosely used term for molecules present in organisms that are essential to one or more typically biological processes, such as cell division, morphogenesis, or development. Biomolecules include large ...
s (biopolymer
Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, cl ...
s such as proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
, peptides
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
and sugars) and large organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
molecules
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ...
(such as polymers, dendrimers
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The ...
and other macromolecules), which tend to be fragile and fragment when ionized by more conventional ionization methods. It is most similar in character to electrospray ionization both in relative softness and the ions produced (although it causes much fewer multiply charged ions). The term was first used in 1985 by Franz Hillenkamp, Michael Karas
Michael Karas (born 1952) is a German physical chemistry scientist and Professor, known for his researches on matrix-assisted laser desorption/ionization (MALDI), a technique in mass spectrometry.
Michael Karas studied Chemistry at the University ...
and their colleagues. These researchers found that the amino acid alanine could be ionized more easily if it was mixed with the amino acid tryptophan and irradiated with a pulsed 266 nm laser. The tryptophan was absorbing the laser energy and helping to ionize the non-absorbing alanine. Peptides up to the 2843 Da peptide melittin could be ionized when mixed with this kind of “matrix”.
The breakthrough for large molecule laser desorption ionization came in 1987 when Koichi Tanaka of Shimadzu Corp. and his co-workers used what they called the “ultra fine metal plus liquid matrix method” that combined 30 nm cobalt particles in glycerol with a 337 nm nitrogen laser for ionization. Using this laser and matrix combination, Tanaka was able to ionize biomolecules as large as the 34,472 Da protein carboxypeptidase-A. Tanaka received one-quarter of the 2002 Nobel Prize in Chemistry for demonstrating that, with the proper combination of laser wavelength and matrix, a protein can be ionized. Karas and Hillenkamp were subsequently able to ionize the 67 kDa protein albumin using a nicotinic acid matrix and a 266 nm laser. Further improvements were realized through the use of a 355 nm laser and the cinnamic acid derivatives ferulic acid, caffeic acid and sinapinic acid as the matrix. The availability of small and relatively inexpensive nitrogen lasers operating at 337 nm wavelength and the first commercial instruments introduced in the early 1990s brought MALDI to an increasing number of researchers. Today, mostly organic matrices are used for MALDI mass spectrometry.
Timeline
19th century
:1886 ::Eugen Goldstein
Eugen Goldstein (; 5 September 1850 – 25 December 1930) was a German physicist. He was an early investigator of discharge tubes, the discoverer of anode rays or canal rays, later identified as positive ions in the gas phase including the hy ...
observes canal rays.
:1898
 :: Wilhelm Wien demonstrates that canal rays can be deflected using strong electric and magnetic fields. He shows that the
:: Wilhelm Wien demonstrates that canal rays can be deflected using strong electric and magnetic fields. He shows that the mass-to-charge ratio
The mass-to-charge ratio (''m''/''Q'') is a physical quantity relating the ''mass'' (quantity of matter) and the ''electric charge'' of a given particle, expressed in units of kilograms per coulomb (kg/C). It is most widely used in the electrody ...
of the particles have opposite polarity and is much larger compared to the electron. He also realizes that the particle mass is similar to the one of hydrogen particle.
:1898
:: J. J. Thomson measures the mass-to-charge ratio
The mass-to-charge ratio (''m''/''Q'') is a physical quantity relating the ''mass'' (quantity of matter) and the ''electric charge'' of a given particle, expressed in units of kilograms per coulomb (kg/C). It is most widely used in the electrody ...
of electrons.
20th century
:1901 :: Walter Kaufmann uses a mass spectrometer to measure the relativistic mass increase of electrons. :1905 :: J. J. Thomson begins his study of positive rays. :1906 ::Thomson is awarded the Nobel Prize in Physics "in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases" :1913 ::Thomson is able to separate particles of differentmass-to-charge ratio
The mass-to-charge ratio (''m''/''Q'') is a physical quantity relating the ''mass'' (quantity of matter) and the ''electric charge'' of a given particle, expressed in units of kilograms per coulomb (kg/C). It is most widely used in the electrody ...
s. He separates the 20Ne and the 22Ne isotopes, and he correctly identifies the ''m/z'' = 11 signal as a doubly charged 22Ne particle.
:1919
:: Francis Aston constructs the first velocity focusing mass spectrograph with mass resolving power of 130.
:1922
::Aston is awarded the Nobel Prize in chemistry "for his discovery, by means of his mass spectrograph, of isotopes, in a large number of non-radioactive elements, and for his enunciation of the whole-number rule."
:1931
 :: Ernest O. Lawrence invents the cyclotron.
:1934
::
:: Ernest O. Lawrence invents the cyclotron.
:1934
::Josef Mattauch
Josef Mattauch (21 November 1895 – 10 August 1976) was a nuclear physicist and chemist. He was known for the development of the Mattauch-Herzog double-focusing mass spectrometer, for his work on the investigation of isotopic abundances using mas ...
and Richard Herzog develop the double-focusing mass spectrograph.
:1936
:: Arthur J. Dempster develops the spark ionization
Spark ionization (also known as spark source ionization) is a method used to produce gas phase ions from a solid sample. The prepared solid sample is vaporized and partially ionized by an intermittent discharge or spark. This technique is primar ...
source.
:1937
::Aston constructs a mass spectrograph with resolving power of 2000.
:1939
::Lawrence receives the Nobel Prize in Physics for the cyclotron.
:1942
::Lawrence develops the Calutron for uranium isotope separation.
:1943
::Westinghouse markets its mass spectrometer and proclaims it to be "A New Electronic Method for fast, accurate gas analysis".
:1946
::William Stephens presents the concept of a time-of-flight mass spectrometer.
:1953
::Wolfgang Paul
Wolfgang Paul (; 10 August 1913 – 7 December 1993) was a German physicist, who co-developed the non-magnetic quadrupole mass filter which laid the foundation for what is now called an ion trap. He shared one-half of the Nobel Prize in Ph ...
and Helmut Steinwedel introduce the quadrupole mass filter.
:1954
::A. J. C. Nicholson (Australia) proposes a hydrogen transfer reaction that will come to be known as the McLafferty rearrangement.
:1959
::Researchers at Dow Chemical
The Dow Chemical Company, officially Dow Inc., is an American multinational chemical corporation headquartered in Midland, Michigan, United States. The company is among the three largest chemical producers in the world.
Dow manufactures plastics ...
interface a gas chromatograph to a mass spectrometer.
:1964
::British Mass Spectrometry Society established as first dedicated mass spectrometry society. It holds its first meeting in 1965 in London.
:1966
::F. H. Field and M. S. B. Munson develop chemical ionization
Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (often ...
.
:1968
:: Malcolm Dole develops electrospray ionization.
:1969
::H. D. Beckey develops field desorption.
:1974
::Comisarow and Marshall develop Fourier Transform Ion Cyclotron Resonance mass spectrometry.
:1976
::Ronald MacFarlane and co-workers develop plasma desorption mass spectrometry.
:1984
:: John Bennett Fenn and co-workers use electrospray to ionize biomolecules.
:1985
::Franz Hillenkamp, Michael Karas and co-workers describe and coin the term matrix-assisted laser desorption ionization
In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of b ...
(MALDI).
:1987
:: Koichi Tanaka uses the “ultra fine metal plus liquid matrix method” to ionize intact proteins.
:1989
::Wolfgang Paul
Wolfgang Paul (; 10 August 1913 – 7 December 1993) was a German physicist, who co-developed the non-magnetic quadrupole mass filter which laid the foundation for what is now called an ion trap. He shared one-half of the Nobel Prize in Ph ...
receives the Nobel Prize in Physics "for the development of the ion trap technique".
:1999
::Alexander Makarov presents the Orbitrap mass spectrometer./ref>
21st century
:2002 John Bennett Fenn and Koichi Tanaka are awarded one-quarter of the Nobel Prize in chemistry each "for the development of soft desorption ionisation methods ... for mass spectrometric analyses of biological macromolecules." :2005 ::Commercialization of Orbitrap MS :2008 :: ASMS Distinguished Contribution in Mass Spectrometry AwardSee also
*Mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is use ...
*History of chemistry
The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis of the various branches of chemistry. Examples include the discovery of fire, e ...
* History of physics
References
Bibliography
*Measuring Mass: From Positive Rays to Proteins by Michael A. Grayson (Editor) () *External links
History of Mass Spectrometry - Pioneers - University of New South Wales Sydney
* ttps://web.archive.org/web/20071108220147/http://masspec.scripps.edu/mshistory/mshistory.php History of Mass Spectrometry - Scripps Institute {{Mass spectrometry Mass spectrometry