Hexaphosphabenzene on:
[Wikipedia]
[Google]
[Amazon]
_
In_geometry,_a_tetrahedron_(plural:_tetrahedra_or_tetrahedrons),_also_known_as_a_triangular_pyramid,_is_a_polyhedron_composed_of_four_triangular_faces,_six_straight__edges,_and_four__vertex_corners._The_tetrahedron_is_the_simplest_of_all_the_o_...
_coordination_geometry_around_the_Cu_center.
The_successful_isolation_of__
Hexaphosphabenzene is a valence isoelectronic analogue of
 Isolation of hexaphosphabenzene was first achieved within a triple-decker
Isolation of hexaphosphabenzene was first achieved within a triple-decker
 If one regards the planar ring as a 6π
If one regards the planar ring as a 6π

To avoid 2(μ,η6-P6).html"_;"title="u(_2(μ,η6-P6)">u([2(μ,η6-P6)2.html"_;"title="sub>2(μ,η6-P6)">u([2(μ,η6-P6)2">sub>2(μ,η6-P6)">u([2(μ,η6-P6)2sup>+_either_as_its_ sub>2(μ,η6-P6)">u([2(μ,η6-P6)2sup>+._However,_analysis_of_crystals_from_this_solution_reveals_a_distorted_Tetrahedral_molecular_geometry">tetrahedral_
In_geometry,_a_tetrahedron_(plural:_tetrahedra_or_tetrahedrons),_also_known_as_a_triangular_pyramid,_is_a_polyhedron_composed_of_four_triangular_faces,_six_straight__edges,_and_four__vertex_corners._The_tetrahedron_is_the_simplest_of_all_the_o_...
_coordination_environment_around_Cu._The_resulting_Cu—P_distances_are_somewhat_shorter_than_their_counterparts_discussed_above._The_coordinating_P—P_bonds_are_a_little_longer,_which_is_attributed_to_less_steric_crowding_in_the_Tetrahedral_molecular_geometry">tetrahedralbenzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
and is expected to have a similar planar structure due to resonance stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
. Although several other allotropes of phosphorus are stable, no evidence for the existence of has been reported. Preliminary ab initio calculations on the trimerisation
In chemistry, a trimer (; ) is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often ...
of leading to the formation of the cyclic were performed, and it was predicted that hexaphosphabenzene would decompose to free with an energy barrier
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
of 13−15.4 kcal mol−1, and would therefore not be observed in the uncomplexed state under normal experimental conditions. The presence of an added solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
, such as ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
, might lead to the formation of intermolecular hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
s which may block the destabilizing interaction between phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ear ...
lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s and consequently stabilize . The moderate barrier
A barrier or barricade is a physical structure which blocks or impedes something.
Barrier may also refer to:
Places
* Barrier, Kentucky, a community in the United States
* Barrier, Voerendaal, a place in the municipality of Voerendaal, Netherl ...
suggests that hexaphosphabenzene could be synthesized from a +2+2cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
of three molecules. Currently, this is a synthetic endeavour which remains to be conquered.
Synthesis
 Isolation of hexaphosphabenzene was first achieved within a triple-decker
Isolation of hexaphosphabenzene was first achieved within a triple-decker sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic derivat ...
in 1985 by Scherer et al. Amber coloured, air-stable crystals of sub>2(μ,η6-P6)are formed by reaction of with excess in dimethylbenzene, albeit with a yield of approximately 1%. The crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pat ...
of this complex is a centrosymmetric
In crystallography, a centrosymmetric point group contains an inversion center as one of its symmetry elements. In such a point group, for every point (x, y, z) in the unit cell there is an indistinguishable point (-x, -y, -z). Such point groups ...
molecule, and both five-membered rings as well as the central bridge-ligand ring are planar and parallel. The average P–P distance for the hexaphosphabenzene within this complex is 2.170 Å.
Thirty years later, Fleischmann et al. improved the synthetic yield of sub>2(μ,η6-P6)up to 64%. This was achieved by increasing the reaction temperature of the thermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
of with to approximately 205 °C in boiling diisopropylbenzene
In organic chemistry, the diisopropylbenzenes constitute a group of aromatic hydrocarbons, whose chemical structure consists of a benzene ring () with two isopropyl groups () as substituents. Through their different arrangement, they form three st ...
, thus favouring the formation of sub>2(μ,η6-P6)as the thermodynamic product.
Several analogues of this triple‐decker complex
Complex commonly refers to:
* Complexity, the behaviour of a system whose components interact in multiple ways so possible interactions are difficult to describe
** Complex system, a system composed of many components which may interact with each ...
where the coordinating metal and η5-ligand has been varied have also been reported. These include triple‐decker complexes for Ti, V, Nb, and W, whereby the synthetic method is still based on the originally reported thermolysis
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is req ...
of with .
Electron count
 If one regards the planar ring as a 6π
If one regards the planar ring as a 6π electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent chem ...
ligand, then sub>2(μ,η6-P6)is a triple-decker sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic derivat ...
with 28 valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
s. If , similar to C6H6, is taken as a 10π electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent chem ...
, a 32 valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
count may be obtained. In most triple-decker complexes with an electron count
Electron counting is a formalism used for classifying compounds and for explaining or predicting electronic structure and chemical bond, bonding. Many rules in chemistry rely on electron-counting:
*Octet rule is used with Lewis structures for mai ...
ranging from 26 to 34, the structure of the middle ring is planar ( sub>2(μ,η6-P6)with M = Mo, Sc, Y, Zr, Hf, V, Nb, Ta, Cr, and W). In the 24 valence electron sub>2(μ,η6-P6)complex, however, a distortion is observed, and the ring is puckered.
Calculations have concluded that completely filled 2a*and 2b* orbitals in 28 valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
complexes lead to a planar symmetrical middle ring. In 26 valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
complexes, the occupancy of either 2a*or 2b* results in in-plane or bisallylic distortions and an asymmetric planar middle ring. The puckering of in 24 valence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
complexes is due to the stabilization of 5a, as well as that conferred by the tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of an ...
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
of Ti in sub>2(μ,η6-P6)
Reactivity

One-electron oxidation
The reactivity of sub>2(μ,η6-P6)towardsilver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
and copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
monocationic salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively cha ...
of the weakly coordinating anion l4sup>− ( EF was studied by Fleischmann et al. in 2015. Addition of a solution of Ag EFor Cu EFto a solution of sub>2(μ,η6-P6)in chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to ...
results in oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of the complex, which can be observed by an immediate colour change from amber to dark teal. The magnetic moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets ...
of the dark teal crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s determined by the Evans NMR method is equal to 1.67 μB, which is consistent with one unpaired electron. Accordingly, sub>2(μ,η6-P6)sup>+ is detected by ESI mass spectrometry.
The crystal structure of the teal product shows that the triple‐decker geometry is retained during the one‐electron oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of sub>2(μ,η6-P6) The Mo—Mo bond length of the sub>2(μ,η6-P6)sup>+ cation is 2.6617(4) Å; almost identical to the bond length determined for the unoxidized species at 2.6463(3) Å. However, the P—P bond lengths are strongly affected by the oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
. While the P1—P1′ and P3—P3′ bonds are elongated, the remaining P—P bonds are shortened compared to the average P—P bond length of about 2.183 Å in the unoxidized species. Therefore, the middle deck of the 27 valence electron sub>2(μ,η6-P6)sup>+ complex can best be described as a bisallylic distorted P6 ligand, intermediate between the 28 valence electron complexes with a perfectly planar symmetrical ring, and those with 26 valence electrons displaying a more amplified in-plane distortion. Density functional theorem (DFT) calculations confirm that this distortion is due to depopulation of the P bonding orbital
In theoretical chemistry, the bonding orbital is used in molecular orbital (MO) theory to describe the attractive interactions between the atomic orbitals of two or more atoms in a molecule. In MO theory, electrons are portrayed to move in waves. ...
s upon oxidation of the triple-decker sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic derivat ...
.
oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of sub>2(μ,η6-P6) further reactions were performed in toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
to decrease the redox potentiacopper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, although a mixture also containing the dark teal oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
product was obtained upon reaction with silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
.
Single‐crystal X‐ray analysis reveals that this product displays a distorted square‐planar coordination environment around the central cation through two side‐on coordinating P—P bonds. The Ag—P distances are approximately 2.6 Å, whereas the Cu—P distances are determined to be approximately 2.4 Å. The P—P bonds are therefore elongated to 2.2694(16) Å and 2.2915(14) Å upon coordination to copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
and silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
, respectively, whilst the remaining P—P bonds are unaffected.
In another experiment Cu EFis treated with sub>2(μ,η6-P6)in pure toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) at ...
and the solution shows the bright orange color of the complex cation u([2(μ,η6-P6)2.html" ;"title="sub>2(μ,η6-P6)">u(sub>2(μ,η6-P6)">u([2(μ,η6-P6)2sup>+._However,_analysis_of_crystals_from_this_solution_reveals_a_distorted__
In_geometry,_a_tetrahedron_(plural:_tetrahedra_or_tetrahedrons),_also_known_as_a_triangular_pyramid,_is_a_polyhedron_composed_of_four_triangular_faces,_six_straight__edges,_and_four__vertex_corners._The_tetrahedron_is_the_simplest_of_all_the_o_...
_coordination_geometry_around_the_Cu_center.
The_successful_isolation_of__sub>2(μ,η6-P6)">u([2(μ,η6-P6)2sup>+._However,_analysis_of_crystals_from_this_solution_reveals_a_distorted_Tetrahedral_molecular_geometry">tetrahedral_
In_geometry,_a_tetrahedron_(plural:_tetrahedra_or_tetrahedrons),_also_known_as_a_triangular_pyramid,_is_a_polyhedron_composed_of_four_triangular_faces,_six_straight__edges,_and_four__vertex_corners._The_tetrahedron_is_the_simplest_of_all_the_o_...
_coordination_environment_around_Cu._The_resulting_Cu—P_distances_are_somewhat_shorter_than_their_counterparts_discussed_above._The_coordinating_P—P_bonds_are_a_little_longer,_which_is_attributed_to_less_steric_crowding_in_the_sub>2(μ,η6-P6)">u([2(μ,η6-P6)2sup>+._However,_analysis_of_crystals_from_this_solution_reveals_a_distorted_Tetrahedral_molecular_geometry">tetrahedral_
In_geometry,_a_tetrahedron_(plural:_tetrahedra_or_tetrahedrons),_also_known_as_a_triangular_pyramid,_is_a_polyhedron_composed_of_four_triangular_faces,_six_straight__edges,_and_four__vertex_corners._The_tetrahedron_is_the_simplest_of_all_the_o_...
_coordination_environment_around_Cu._The_resulting_Cu—P_distances_are_somewhat_shorter_than_their_counterparts_discussed_above._The_coordinating_P—P_bonds_are_a_little_longer,_which_is_attributed_to_less_steric_crowding_in_the_Tetrahedral_molecular_geometry">tetrahedral_or__
coordination environment around Cu. The resulting Cu—P distances are somewhat shorter than their counterparts discussed above. The coordinating P—P bonds are a little longer, which is attributed to less steric crowding in the Tetrahedral molecular geometry">tetrahedral In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
coordination geometry around the Cu center. The successful isolation of u([2(μ,η6-P6)2.html" ;"title="sub>2(μ,η6-P6)">u([2(μ,η6-P6)2">sub>2(μ,η6-P6)">u([2(μ,η6-P6)2sup>+ either as its Tetrahedral molecular geometry">tetrahedral In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
or square‐planar isomer is therefore achievable. Density functional theory">DFT calculations show that the enthalpy for the
tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
to square‐planar isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
is positive for both metals, with the tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
coordination being favored. When entropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodynam ...
is taken into account, small positive values for Cu+ and larger, but negative, values for Ag+ are observed. This means that the tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
geometry is predominant for Cu+, but a significant percentage of the complexes adopt a square‐planar geometry in solution. For Ag+, the equilibrium is shifted significantly to the right side, which is presumably why a tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the o ...
coordination of sub>2(μ,η6-P6)and Ag+ has not yet been observed.
Examination of the crystal packing reveals that these products are layered compounds that crystallize in the monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic s ...
''C''2/''c'' space group
In mathematics, physics and chemistry, a space group is the symmetry group of an object in space, usually in three dimensions. The elements of a space group (its symmetry operations) are the rigid transformations of an object that leave it unchan ...
with alternating negatively charged layers of the EFanions and positively charged layers of isolated ([2(μ,η6-P6)2.html" ;"title="sub>2(μ,η6-P6)">(sub>2(μ,η6-P6)">([2(μ,η6-P6)2sup>+_complexes._The_layers_lie_inside_the_''bc''_plane,_alternate_along_the_''a''_axis,_and_do_not_form_a_two‐dimensional_network.
2(μ,η6-P6).html"_;"title="sub>2(μ,η6-P6)">sub>2(μ,η6-P6)with_Tl_EFin_chloroform_
Chloroform,_or_trichloromethane,_is_an_organic_compound_with_chemical_formula,_formula_Carbon,_CHydrogen,_HChlorine,_Cl3_and_a_common_organic_solvent._It_is_a_colorless,_strong-smelling,_dense_liquid_produced_on_a_large_scale_as_a_precursor_to__...
_gives_an_immediate_color_change_from_amber_to_a_deep_red._The_crystal_structure_reveals_a_Trigonal_pyramidal_molecular_geometry.html" "title="sub>2(μ,η6-P6)2">sub>2(μ,η6-P6)">([2(μ,η6-P6)2sup>+ complexes. The layers lie inside the ''bc'' plane, alternate along the ''a'' axis, and do not form a two‐dimensional network.
__Tl_
Tl EF
The treatment of sub>2(μ,η6-P6)with Tl EFinchloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to ...
gives an immediate color change from amber to a deep red. The crystal structure reveals a Trigonal pyramidal molecular geometry">trigonal pyramidal
In chemistry, a trigonal pyramid is a molecular geometry with one atom at the apex and three atoms at the corners of a trigonal base, resembling a tetrahedron (not to be confused with the tetrahedral geometry). When all three atoms at the corner ...coordination of the thallium cation, Tl+, by three side‐on coordinating P—P bonds of the P6 ligands. Two of these P6 ligands show shorter and uniform Tl—P distances of 3.2–3.3 Å with P—P bonds elongated to about 2.22 Å, whilst the third unit shows an unsymmetrical coordination with long Tl—P distances of approximately 3.42 and 3.69 Å and no P—P bond elongation. Although the environment of Tl+ is distinctly different from that of Cu+ and Ag+, their structures are related by the two‐dimensional coordination network that propagates inside the ''bc'' plane. Crucially, whilst Cu+ and Ag+ form layered structures with isolated ([2(μ,η6-P6)2.html" ;"title="sub>2(μ,η6-P6)">([2(μ,η6-P6)2">sub>2(μ,η6-P6)">([2(μ,η6-P6)2sup>+ complex cations, there is a statistical distribution of the Tl+ cations inside the two‐dimensional coordination, which shows further interconnection of the P6 ligands to form an extended 2D network that could be regarded as a supramolecular analogue of
graphene
Graphene () is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure.
.
Jahn-Teller distortion
 Despite the triple-decker
Despite the triple-decker sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic derivat ...
2(μ,η6-P6) containing a demonstrably planar P6 ring with equal P—P bond lengths, theoretical calculations reveal that there are at least 7 non-planar P6 isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
s lower in energy than the planar benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
-like D6h structure. In increasing order of energy these are: benzvalene, prismane, chair, Dewar benzene, bicyclopropenyl, distorted benzene, and benzene. A pseudo Jahn-Teller effect (PJT) is responsible for distortion of the D6h
A pseudo Jahn-Teller effect (PJT) is responsible for distortion of the D6h benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
-like structure into the D2 structure, which occurs along the e2u doubly degenerate mode
Mode ( la, modus meaning "manner, tune, measure, due measure, rhythm, melody") may refer to:
Arts and entertainment
* '' MO''D''E (magazine)'', a defunct U.S. women's fashion magazine
* ''Mode'' magazine, a fictional fashion magazine which is ...
as a result of vibronic coupling
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" a ...
of the HOMO
''Homo'' () is the genus that emerged in the (otherwise extinct) genus ''Australopithecus'' that encompasses the extant species ''Homo sapiens'' ( modern humans), plus several extinct species classified as either ancestral to or closely relate ...
− 1 (e2g) and LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
(e2u): e2g ⊗ e2u = a1u ⊕ a2u ⊕ e2u. The distorted structure is calculated to lie just 2.7 kcal mol−1 lower in energy than the D6h structure. If the uncomplexed structure were to be successfully synthesized, the aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturate ...
of the benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
-like P6 structure would not be sufficient to stabilize the planar geometry, and the PJT effect would result in distortion of the ring.
Isomers
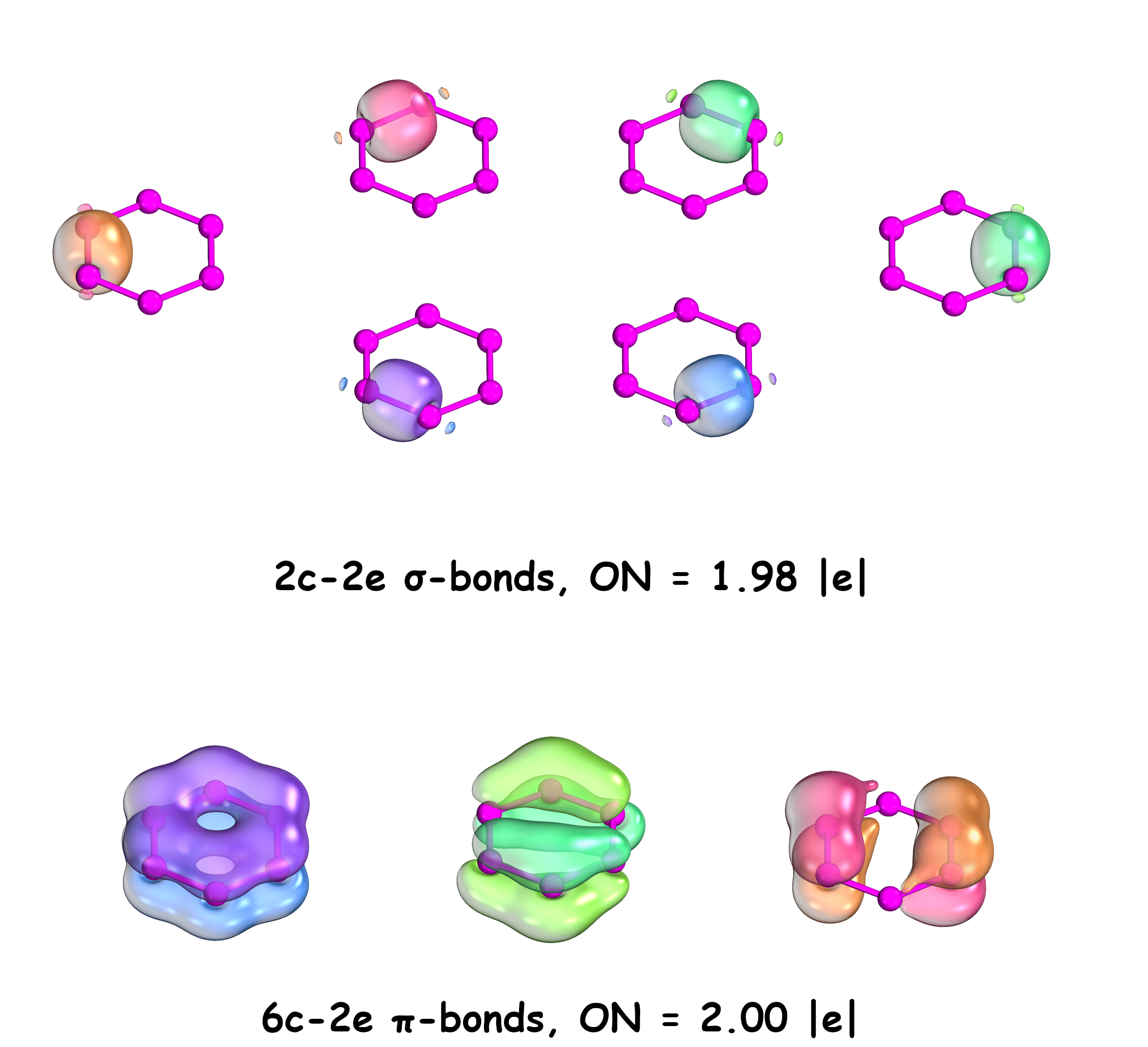 Adaptive Natural Density Partitioning (AdNDP) is a theoretical tool developed by Alexander Boldyrev that is based on the concept of the electron pair as the main element of chemical bonding models. It can therefore recover Lewis bonding elements such as 1c–2e core electrons and lone pairs, 2c–2e objects which are two-center two-electron bonds, as well as delocalized many-center bonding elements with respect to aromaticity.
The AdNDP analysis of the seven representative low-lying P6 structures reveal that these are well described by the classical Lewis model. A
Adaptive Natural Density Partitioning (AdNDP) is a theoretical tool developed by Alexander Boldyrev that is based on the concept of the electron pair as the main element of chemical bonding models. It can therefore recover Lewis bonding elements such as 1c–2e core electrons and lone pairs, 2c–2e objects which are two-center two-electron bonds, as well as delocalized many-center bonding elements with respect to aromaticity.
The AdNDP analysis of the seven representative low-lying P6 structures reveal that these are well described by the classical Lewis model. A lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
on each phosphorus atom, a two-center-two-electron (2c–2e) σ-bond in every pair of adjacent P atoms, and an additional 2c–2e π-bond between adjacent 2-coordinated P atoms are found, with occupation numbers (ON) of all these bonding elements above 1.92 , e, .
The chemical bonding in the chair structure is unusual. Based on fragment orbital analysis, it was concluded that two linkages between the two P3 fragments are of the one-electron hemibond type. The AdNDP analysis reveals a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
on each P atom and six 2c–2e P—P σ-bonds. One 3c–2e π-bond in every P3 triangle was revealed with the user-directed form of the AdNDP analysis, as well as a 4c–2e bond responsible for bonding between the two P3 triangle, confirming that this isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
cannot be represented by a single Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the chemical bonding, bonding between atoms of a molecule, as well as the lone pairs ...
, and requires a resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
of two Lewis structure
Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the chemical bonding, bonding between atoms of a molecule, as well as the lone pairs ...
s, or can be described by a single formula with delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
bonding elements.
Both the D6h benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
-like structure, as well as the D2 isomer of P6 is similar to the reported AdNDP bonding pattern of the C6H6 benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
molecule: 2c–2e σ-bond and lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
s, as well as delocalized
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly dif ...
6c-2e π-bonds. The distortion due to the PJT effect therefore does not significantly disturb the bonding picture.Suppression

 The planar P6 hexagonal structure D6h is a second-order saddle point due to the pseudo-Jahn-Teller effect (PJT), which leads to the D2 distorted structure. Upon
The planar P6 hexagonal structure D6h is a second-order saddle point due to the pseudo-Jahn-Teller effect (PJT), which leads to the D2 distorted structure. Upon sandwich complex
In organometallic chemistry, a sandwich compound is a chemical compound featuring a metal bound by haptic, covalent bonds to two arene (ring) ligands. The arenes have the formula , substituted derivatives (for example ) and heterocyclic derivat ...
formation the PJT effect is suppressed due to filling of the unoccupied molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s involved in vibronic coupling
Vibronic coupling (also called nonadiabatic coupling or derivative coupling) in a molecule involves the interaction between electronic and nuclear vibrational motion. The term "vibronic" originates from the combination of the terms "vibrational" a ...
in P6 with electron pairs of Mo atoms. Specifically, from molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
analysis it was determined that, upon complex formation, the LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
in the isolated P6 structure is now occupied in the triple-decker complex as a result of the appreciable δ-type M → L back-donation mechanism from the occupied dx2–y2 and dxy atomic orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any spe ...
s of the Mo atom into the partially antibonding
In chemical bonding theory, an antibonding orbital is a type of molecular orbital that weakens the chemical bond between two atoms and helps to raise the energy of the molecule relative to the separated atoms. Such an orbital has one or more no ...
π molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s of P6, thus restoring the high symmetry and planarity of P6.
References
{{reflist Phosphorus Sandwich compounds Inorganic chemistry Solid-state chemistry Hypothetical chemical compounds Aromatic compounds Six-membered rings