|
Supramolecular Assembly
In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules (e.g., a DNA double helix or an inclusion compound), or a defined number of stoichiometrically interacting molecules within a quaternary complex, it is more often used to denote larger complexes composed of indefinite numbers of molecules that form sphere-, rod-, or sheet-like species. Colloids, liquid crystals, biomolecular condensates, micelles, liposomes and biological membranes are examples of supramolecular assemblies, and their realm of study is known as supramolecular chemistry. The dimensions of supramolecular assemblies can range from nanometers to micrometers. Thus they allow access to nanoscale objects using a bottom-up approach in far fewer steps than a single molecule of similar dimensions. The process by which a supramolecular assembly forms is called molecular self-assembly. Some try to dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
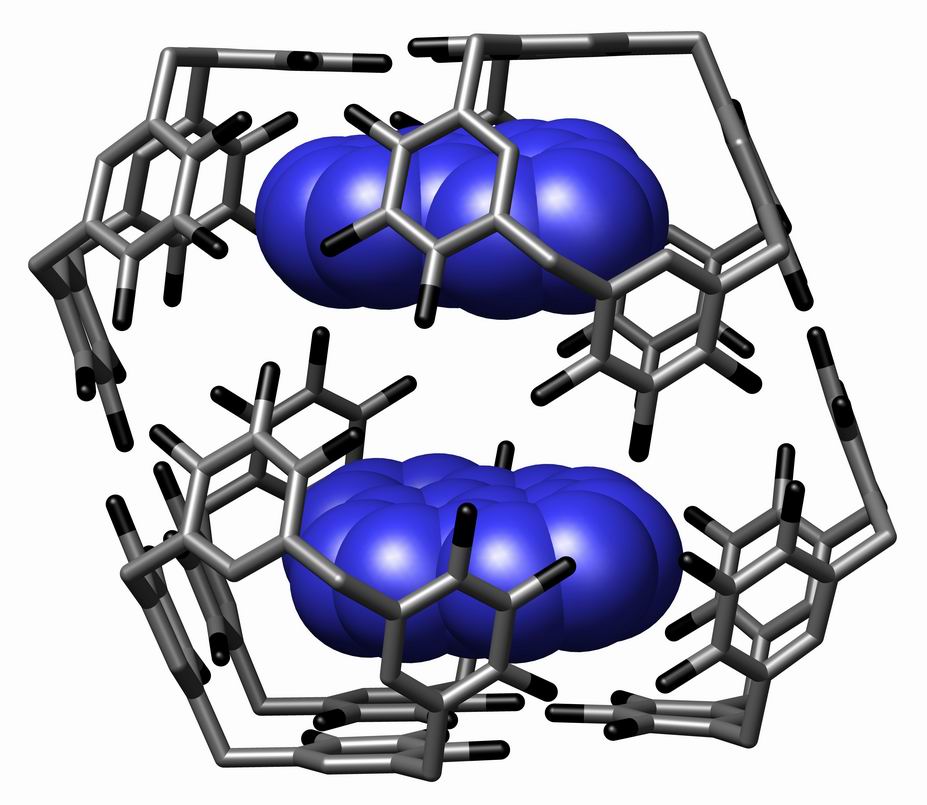
Host Guest Complex Nanocapsule Science Year2005 Vol309 Page2037
A host is a person responsible for guests at an event or for providing hospitality during it. Host may also refer to: Places * Host, Pennsylvania, a village in Berks County People *Jim Host (born 1937), American businessman * Michel Host (1942–2021), French writer *"Host", an author abbreviation in botany for Nicolaus Thomas Host Arts, entertainment, and media Fictional entities * Hosts (''World of Darkness''), fictional characters in game ''Werewolf: The Forsaken'' *Hosts, alien invaders and overlords in the TV series ''Colony'' * Armies and hosts of Middle-earth warfare, fictional entities in J.R.R. Tolkien's works *Avenging Host, a group of characters in Marvel Comics' ''Earth X'' series of comic books * Rutan Host, fictional aliens from ''Doctor Who'' Film * ''Host'' (film), a 2020 horror film directed by Rob Savage Literature *''Host'', the third novel in the Rogue Mage series by Faith Hunter *''Host'', a 1993 book by Peter James * ''Hosts'' (novel), a 2001 bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self-organization
Self-organization, also called spontaneous order in the social sciences, is a process where some form of overall order arises from local interactions between parts of an initially disordered system. The process can be spontaneous when sufficient energy is available, not needing control by any external agent. It is often triggered by seemingly random fluctuations, amplified by positive feedback. The resulting organization is wholly decentralized, distributed over all the components of the system. As such, the organization is typically robust and able to survive or self-repair substantial perturbation. Chaos theory discusses self-organization in terms of islands of predictability in a sea of chaotic unpredictability. Self-organization occurs in many physical, chemical, biological, robotic, and cognitive systems. Examples of self-organization include crystallization, thermal convection of fluids, chemical oscillation, animal swarming, neural circuits, and black markets. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted , where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the second-row elements nitrogen (N), oxygen (O), and fluorine (F). Hydrogen bonds can be intermolecular (occurring between separate molecules) or intramolecular (occurring among parts of the same molecule). The energy of a hydrogen bond depends on the geometry, the environment, and the nature of the specific donor and acceptor atoms and can vary between 1 and 40 kcal/mol. This makes them somewhat stronger than a van der Waals interaction, and weaker than fully covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bonds In (a) DNA Duplex Formation And (b) Protein β-sheet Structure
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,00 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Williamson Ether Synthesis
The Williamson ether synthesis is an organic reaction, forming an ether from an organohalide and a deprotonated alcohol ( alkoxide). This reaction was developed by Alexander Williamson in 1850. Typically it involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. This reaction is important in the history of organic chemistry because it helped prove the structure of ethers. The general reaction mechanism is as follows: An example is the reaction of sodium ethoxide with chloroethane to form diethyl ether and sodium chloride: :C2H5Cl + C2H5ONa -> C2H5OC2H5 + NaCl Mechanism The Williamson ether reaction follows an SN2 bimolecular nucleophilic substitution mechanism. In an SN2 reaction mechanism there is a backside attack of an electrophile by a nucleophile and it occurs in a concerted mechanism (happens all at once). In order for the SN2 reaction to take place there must be a good leaving group which is strongly electronegative, commonly a hali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
18-crown-6
18-Crown-6 is an organic compound with the formula [C2H4O]6 and the IUPAC name of 1,4,7,10,13,16-hexaoxacyclooctadecane. It is a white, hygroscopic crystalline solid with a low melting point. Like other crown ethers, 18-crown-6 functions as a ligand for some metal cations with a particular affinity for potassium cations (binding constant in methanol: 106 M−1). The point group of 18-crown-6 is S6. The dipole moment of 18-crown-6 varies in different solvent and under different temperature. Under 25 °C, the dipole moment of 18-crown-6 is in cyclohexane and in benzene. The synthesis of the crown ethers led to the awarding of the Nobel Prize in Chemistry to Charles J. Pedersen. Synthesis This compound is prepared by a modified Williamson ether synthesis in the presence of a templating cation: It can be also prepared by the oligomerization of ethylene oxide: :(CH2OCH2CH2Cl)2 + (CH2OCH2CH2OH)2 + 2 KOH → (CH2CH2O)6 + 2 KCl + 2 H2O It can be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crown Ethers
In organic chemistry, crown ethers are cyclic chemical compounds that consist of a Ring (chemistry), ring containing several ether groups (). The most common crown ethers are cyclic oligomers of ethylene oxide, the repeating unit being ethyleneoxy, i.e., . Important members of this series are the tetramer (''n'' = 4), the pentamer (''n'' = 5), and the hexamer (''n'' = 6). The term "crown" refers to the resemblance between the structure of a crown ether bound to a cation, and a crown (headgear), crown sitting on a person's head. The first number in a crown ether's name refers to the number of atoms in the cycle, and the second number refers to the number of those atoms that are oxygen. Crown ethers are much broader than the oligomers of ethylene oxide; an important group are derived from catechol. Crown ethers strongly bind certain cations, forming complex (chemistry), complexes. The oxygen atoms are well situated to coordinate with a cation locate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charles J
Charles is a masculine given name predominantly found in English and French speaking countries. It is from the French form ''Charles'' of the Proto-Germanic name (in runic alphabet) or ''*karilaz'' (in Latin alphabet), whose meaning was "free man". The Old English descendant of this word was '' Ċearl'' or ''Ċeorl'', as the name of King Cearl of Mercia, that disappeared after the Norman conquest of England. The name was notably borne by Charlemagne (Charles the Great), and was at the time Latinized as ''Karolus'' (as in ''Vita Karoli Magni''), later also as '' Carolus''. Some Germanic languages, for example Dutch and German, have retained the word in two separate senses. In the particular case of Dutch, ''Karel'' refers to the given name, whereas the noun ''kerel'' means "a bloke, fellow, man". Etymology The name's etymology is a Common Germanic noun ''*karilaz'' meaning "free man", which survives in English as churl (< Old English ''ċeorl''), which developed its depr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Ions
Transition or transitional may refer to: Mathematics, science, and technology Biology * Transition (genetics), a point mutation that changes a purine nucleotide to another purine (A ↔ G) or a pyrimidine nucleotide to another pyrimidine (C ↔ T) * Transitional fossil, any fossilized remains of a lifeform that exhibits the characteristics of two distinct taxonomic groups * A phase during childbirth contractions during which the cervix completes its dilation Gender and sex * Gender transitioning, the process of changing one's gender presentation to accord with one's internal sense of one's gender – the idea of what it means to be a man or woman * Sex reassignment therapy, the physical aspect of a gender transition Physics * Phase transition, a transformation of the state of matter; for example, the change between a solid and a liquid, between liquid and gas or between gas and plasma * Quantum phase transition, a phase transformation between different quantum phases * Quantum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Ions
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_self-organization2.jpg)





