Heterogeneous Catalysts on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, heterogeneous catalysis is
 Most metal surface reactions occur by chain propagation in which catalytic intermediates are cyclically produced and consumed. Two main mechanisms for surface reactions can be described for A + B → C.
* Langmuir–Hinshelwood mechanism: The reactant molecules, A and B, both adsorb to the catalytic surface. While adsorbed to the surface, they combine to form product C, which then desorbs.
* Eley–Rideal mechanism: One reactant molecule, A, adsorbs to the catalytic surface. Without adsorbing, B reacts with absorbed A to form C, that then desorbs from the surface.
Most heterogeneously catalyzed reactions are described by the Langmuir–Hinshelwood model.
In heterogeneous catalysis, reactants
Most metal surface reactions occur by chain propagation in which catalytic intermediates are cyclically produced and consumed. Two main mechanisms for surface reactions can be described for A + B → C.
* Langmuir–Hinshelwood mechanism: The reactant molecules, A and B, both adsorb to the catalytic surface. While adsorbed to the surface, they combine to form product C, which then desorbs.
* Eley–Rideal mechanism: One reactant molecule, A, adsorbs to the catalytic surface. Without adsorbing, B reacts with absorbed A to form C, that then desorbs from the surface.
Most heterogeneously catalyzed reactions are described by the Langmuir–Hinshelwood model.
In heterogeneous catalysis, reactants
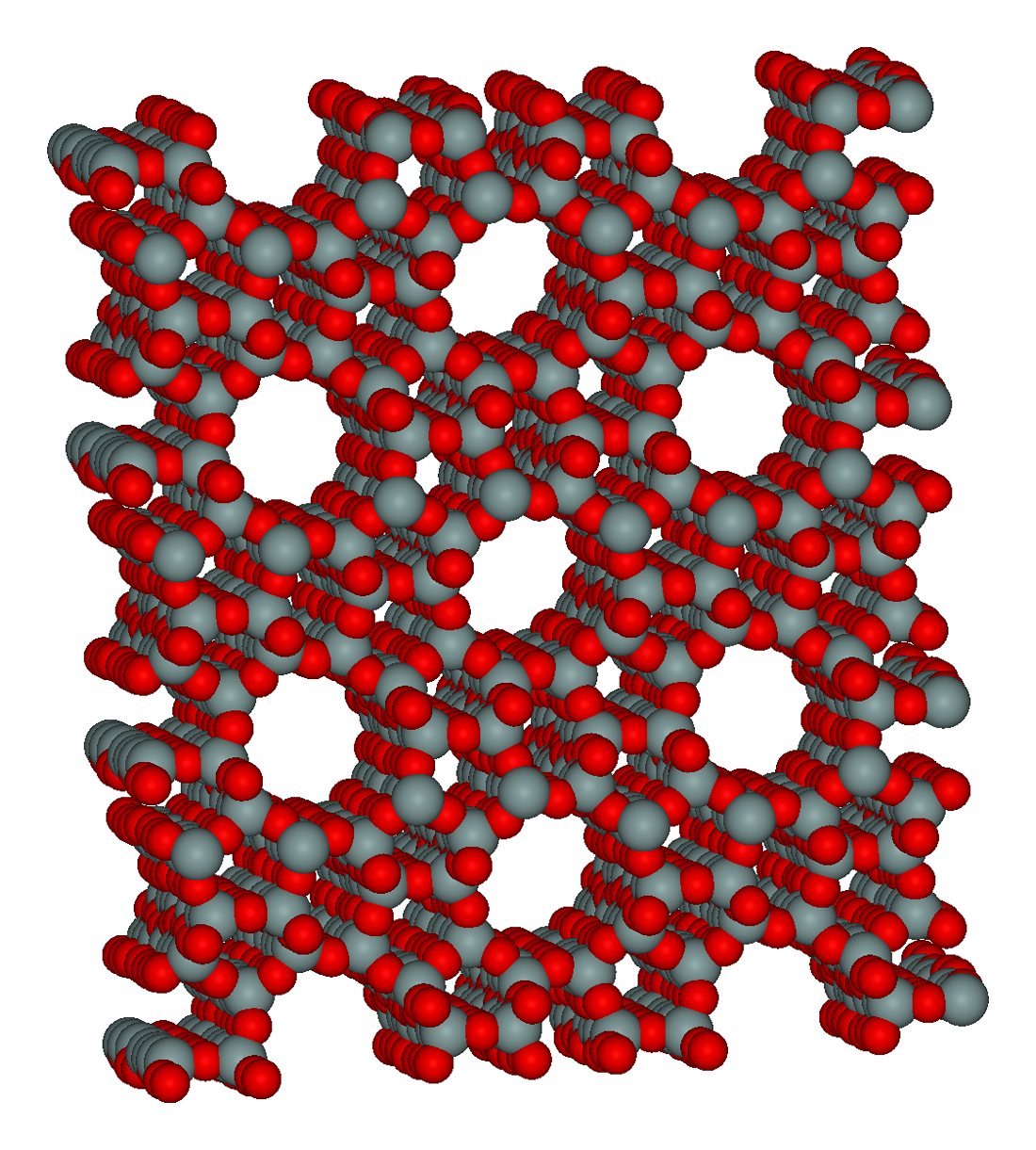 Catalysts are not active towards reactants across their entire surface; only specific locations possess catalytic activity, called active sites. The surface area of a solid catalyst has a strong influence on the number of available active sites. In industrial practice, solid catalysts are often porous to maximize surface area, commonly achieving 50–400 m2/g. Some mesoporous silicates, such as the MCM-41, have surface areas greater than 1000 m2/g. Porous materials are cost effective due to their high surface area-to-mass ratio and enhanced catalytic activity.
In many cases, a solid catalyst is dispersed on a supporting material to increase surface area (spread the number of active sites) and provide stability. Usually catalyst supports are inert, high melting point materials, but they can also be catalytic themselves. Most catalyst supports are porous (frequently carbon, silica, zeolite, or alumina-based) and chosen for their high surface area-to-mass ratio. For a given reaction, porous supports must be selected such that reactants and products can enter and exit the material.
Often, substances are intentionally added to the reaction feed or on the catalyst to influence catalytic activity, selectivity, and/or stability. These compounds are called promoters. For example, alumina (Al2O3) is added during ammonia synthesis to providing greater stability by slowing sintering processes on the Fe-catalyst.
Sabatier principle can be considered one of the cornerstones of modern theory of catalysis. Sabatier principle states that the surface-adsorbates interaction has to be an optimal amount: not too weak to be inert toward the reactants and not too strong to poison the surface and avoid desorption of the products. The statement that the surface-adsorbate interaction has to be an optimum, is a qualitative one. Usually the number of adsorbates and transition states associated with a chemical reaction is a large number, thus the
Catalysts are not active towards reactants across their entire surface; only specific locations possess catalytic activity, called active sites. The surface area of a solid catalyst has a strong influence on the number of available active sites. In industrial practice, solid catalysts are often porous to maximize surface area, commonly achieving 50–400 m2/g. Some mesoporous silicates, such as the MCM-41, have surface areas greater than 1000 m2/g. Porous materials are cost effective due to their high surface area-to-mass ratio and enhanced catalytic activity.
In many cases, a solid catalyst is dispersed on a supporting material to increase surface area (spread the number of active sites) and provide stability. Usually catalyst supports are inert, high melting point materials, but they can also be catalytic themselves. Most catalyst supports are porous (frequently carbon, silica, zeolite, or alumina-based) and chosen for their high surface area-to-mass ratio. For a given reaction, porous supports must be selected such that reactants and products can enter and exit the material.
Often, substances are intentionally added to the reaction feed or on the catalyst to influence catalytic activity, selectivity, and/or stability. These compounds are called promoters. For example, alumina (Al2O3) is added during ammonia synthesis to providing greater stability by slowing sintering processes on the Fe-catalyst.
Sabatier principle can be considered one of the cornerstones of modern theory of catalysis. Sabatier principle states that the surface-adsorbates interaction has to be an optimal amount: not too weak to be inert toward the reactants and not too strong to poison the surface and avoid desorption of the products. The statement that the surface-adsorbate interaction has to be an optimum, is a qualitative one. Usually the number of adsorbates and transition states associated with a chemical reaction is a large number, thus the
 Some large-scale industrial processes incorporating heterogeneous catalysts are listed below.
Some large-scale industrial processes incorporating heterogeneous catalysts are listed below.

catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
where the phase of catalysts differs from that of the reactants
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
or product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Produ ...
s. The process contrasts with homogeneous catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysi ...
where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural ...
, liquid, and gas
Gas is one of the four fundamental states of matter (the others being solid, liquid, and plasma).
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or ...
components, but also immiscible mixtures (e.g. oil
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) & lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturated ...
and water
Water (chemical formula ) is an Inorganic compound, inorganic, transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living ...
), or anywhere an interface is present.
Heterogeneous catalysis typically involves solid phase catalysts and gas phase reactants. In this case, there is a cycle of molecular adsorption, reaction, and desorption occurring at the catalyst surface. Thermodynamics, mass transfer, and heat transfer influence the rate (kinetics) of reaction.
Heterogeneous catalysis is very important because it enables faster, large-scale production and the selective product formation. Approximately 35% of the world's GDP is influenced by catalysis. The production of 90% of chemicals (by volume) is assisted by solid catalysts. The chemical and energy industries rely heavily on heterogeneous catalysis. For example, the Haber–Bosch process
The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and C ...
uses metal-based catalysts in the synthesis of ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
, an important component in fertilizer; 144 million tons of ammonia were produced in 2016.
Adsorption
Adsorption
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ...
is an essential step in heterogeneous catalysis. Adsorption is the process by which a gas (or solution) phase molecule (the adsorbate) binds to solid (or liquid) surface atoms (the adsorbent). The reverse of adsorption is desorption
Desorption is the physical process where a previously adsorbed substance is released from a surface. This happens when a molecule gains enough energy to overcome the activation barrier of the bounding energy that keeps it in the surface.
There ...
, the adsorbate splitting from adsorbent. In a reaction facilitated by heterogeneous catalysis, the catalyst is the adsorbent and the reactants are the adsorbate.
Types of adsorption
Two types of adsorption are recognized:physisorption
Physisorption, also called physical adsorption, is a process in which the electronic structure of the atom or molecule is barely perturbed upon adsorption.
Overview
The fundamental interacting force of physisorption is Van der Waals force. Even ...
, weakly bound adsorption, and chemisorption
Chemisorption is a kind of adsorption which involves a chemical reaction between the surface and the adsorbate. New chemical bonds are generated at the adsorbent surface. Examples include macroscopic phenomena that can be very obvious, like cor ...
, strongly bound adsorption. Many processes in heterogeneous catalysis lie between the two extremes. The Lennard-Jones model provides a basic framework for predicting molecular interactions as a function of atomic separation.
Physisorption
In physisorption, a molecule becomes attracted to the surface atoms viavan der Waals force
In molecular physics, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and th ...
s. These include dipole-dipole interactions, induced dipole interactions, and London dispersion forces. Note that no chemical bonds are formed between adsorbate and adsorbent, and their electronic states remain relatively unperturbed. Typical energies for physisorption are from 3 to 10 kcal/mol. In heterogeneous catalysis, when a reactant molecule physisorbs to a catalyst, it is commonly said to be in a precursor state, an intermediate energy state before chemisorption, a more strongly bound adsorption. From the precursor state, a molecule can either undergo chemisorption, desorption, or migration across the surface. The nature of the precursor state can influence the reaction kinetics.
Chemisorption
When a molecule approaches close enough to surface atoms such that their electron clouds overlap, chemisorption can occur. In chemisorption, the adsorbate and adsorbent share electrons signifying the formation ofchemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s. Typical energies for chemisorption range from 20 to 100 kcal/mol. Two cases of chemisorption are:
* Molecular adsorption: the adsorbate remains intact. An example is alkene binding by platinum.
* Dissociation adsorption: one or more bonds break concomitantly with adsorption. In this case, the barrier to dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts ...
affects the rate of adsorption. An example of this is the binding of H2 to a metal catalyst, where the H-H bond is broken upon adsorption.
Surface reactions
 Most metal surface reactions occur by chain propagation in which catalytic intermediates are cyclically produced and consumed. Two main mechanisms for surface reactions can be described for A + B → C.
* Langmuir–Hinshelwood mechanism: The reactant molecules, A and B, both adsorb to the catalytic surface. While adsorbed to the surface, they combine to form product C, which then desorbs.
* Eley–Rideal mechanism: One reactant molecule, A, adsorbs to the catalytic surface. Without adsorbing, B reacts with absorbed A to form C, that then desorbs from the surface.
Most heterogeneously catalyzed reactions are described by the Langmuir–Hinshelwood model.
In heterogeneous catalysis, reactants
Most metal surface reactions occur by chain propagation in which catalytic intermediates are cyclically produced and consumed. Two main mechanisms for surface reactions can be described for A + B → C.
* Langmuir–Hinshelwood mechanism: The reactant molecules, A and B, both adsorb to the catalytic surface. While adsorbed to the surface, they combine to form product C, which then desorbs.
* Eley–Rideal mechanism: One reactant molecule, A, adsorbs to the catalytic surface. Without adsorbing, B reacts with absorbed A to form C, that then desorbs from the surface.
Most heterogeneously catalyzed reactions are described by the Langmuir–Hinshelwood model.
In heterogeneous catalysis, reactants diffuse
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
from the bulk fluid phase to adsorb to the catalyst surface. The adsorption site is not always an active catalyst site, so reactant molecules must migrate across the surface to an active site. At the active site, reactant molecules will react to form product molecule(s) by following a more energetically facile path through catalytic intermediates (see figure to the right). The product molecules then desorb from the surface and diffuse away. The catalyst itself remains intact and free to mediate further reactions. Transport phenomena such as heat and mass transfer, also play a role in the observed reaction rate.
Catalyst design
 Catalysts are not active towards reactants across their entire surface; only specific locations possess catalytic activity, called active sites. The surface area of a solid catalyst has a strong influence on the number of available active sites. In industrial practice, solid catalysts are often porous to maximize surface area, commonly achieving 50–400 m2/g. Some mesoporous silicates, such as the MCM-41, have surface areas greater than 1000 m2/g. Porous materials are cost effective due to their high surface area-to-mass ratio and enhanced catalytic activity.
In many cases, a solid catalyst is dispersed on a supporting material to increase surface area (spread the number of active sites) and provide stability. Usually catalyst supports are inert, high melting point materials, but they can also be catalytic themselves. Most catalyst supports are porous (frequently carbon, silica, zeolite, or alumina-based) and chosen for their high surface area-to-mass ratio. For a given reaction, porous supports must be selected such that reactants and products can enter and exit the material.
Often, substances are intentionally added to the reaction feed or on the catalyst to influence catalytic activity, selectivity, and/or stability. These compounds are called promoters. For example, alumina (Al2O3) is added during ammonia synthesis to providing greater stability by slowing sintering processes on the Fe-catalyst.
Sabatier principle can be considered one of the cornerstones of modern theory of catalysis. Sabatier principle states that the surface-adsorbates interaction has to be an optimal amount: not too weak to be inert toward the reactants and not too strong to poison the surface and avoid desorption of the products. The statement that the surface-adsorbate interaction has to be an optimum, is a qualitative one. Usually the number of adsorbates and transition states associated with a chemical reaction is a large number, thus the
Catalysts are not active towards reactants across their entire surface; only specific locations possess catalytic activity, called active sites. The surface area of a solid catalyst has a strong influence on the number of available active sites. In industrial practice, solid catalysts are often porous to maximize surface area, commonly achieving 50–400 m2/g. Some mesoporous silicates, such as the MCM-41, have surface areas greater than 1000 m2/g. Porous materials are cost effective due to their high surface area-to-mass ratio and enhanced catalytic activity.
In many cases, a solid catalyst is dispersed on a supporting material to increase surface area (spread the number of active sites) and provide stability. Usually catalyst supports are inert, high melting point materials, but they can also be catalytic themselves. Most catalyst supports are porous (frequently carbon, silica, zeolite, or alumina-based) and chosen for their high surface area-to-mass ratio. For a given reaction, porous supports must be selected such that reactants and products can enter and exit the material.
Often, substances are intentionally added to the reaction feed or on the catalyst to influence catalytic activity, selectivity, and/or stability. These compounds are called promoters. For example, alumina (Al2O3) is added during ammonia synthesis to providing greater stability by slowing sintering processes on the Fe-catalyst.
Sabatier principle can be considered one of the cornerstones of modern theory of catalysis. Sabatier principle states that the surface-adsorbates interaction has to be an optimal amount: not too weak to be inert toward the reactants and not too strong to poison the surface and avoid desorption of the products. The statement that the surface-adsorbate interaction has to be an optimum, is a qualitative one. Usually the number of adsorbates and transition states associated with a chemical reaction is a large number, thus the optimum
Mathematical optimization (alternatively spelled ''optimisation'') or mathematical programming is the selection of a best element, with regard to some criterion, from some set of available alternatives. It is generally divided into two subfi ...
has to be found in a many-dimensional space. Catalyst design in such a many-dimensional space is not a computationally viable task. Additionally, such optimization process would be far from intuitive. Scaling relations are used to decrease the dimensionality of the space of catalyst design. Such relations are correlations among adsorbates binding energies (or among adsorbate binding energies and transition states also known as BEP relations) that are "similar enough" e.g., OH versus OOH scaling. Applying scaling relations to the catalyst design problems greatly reduces the space dimensionality (sometimes to as small as 1 or 2). One can also use micro-kinetic modeling based on such scaling relations to take into account the kinetics associated with adsorption, reaction and desorption of molecules under specific pressure or temperature conditions. Such modeling then leads to well-known volcano-plots at which the optimum qualitatively described by the Sabatier principle is referred to as the "top of the volcano". Scaling relations can be used not only to connect the energetics of radical surface-adsorbed groups (e.g., O*,OH*), but also to connect the energetics of closed-shell
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
molecules among each other or to the counterpart radical adsorbates. A recent challenge for researchers in catalytic sciences is to "break" the scaling relations. The correlations which are manifested in the scaling relations confine the catalyst design space, preventing one from reaching the "top of the volcano". Breaking scaling relations can refer to either designing surfaces or motifs that do not follow a scaling relation, or ones that follow a different scaling relation (than the usual relation for the associated adsorbates) in the right direction: one that can get us closer to the top of the reactivity volcano. In addition to studying catalytic reactivity, scaling relations can be used to study and screen materials for selectivity toward a special product. There are special combination of binding energies that favor specific products over the others. Sometimes a set of binding energies that can change the selectivity toward a specific product "scale" with each other, thus to improve the selectivity one has to break some scaling relations; an example of this is the scaling between methane and methanol oxidative activation energies that leads to the lack of selectivity in direct conversion of methane to methanol.
Catalyst deactivation
Catalyst deactivation is defined as a loss in catalytic activity and/or selectivity over time. Substances that decrease reaction rate are called poisons. Poisons chemisorb to catalyst surface and reduce the number of available active sites for reactant molecules to bind to. Common poisons include Group V, VI, and VII elements (e.g. S, O, P, Cl), some toxic metals (e.g. As, Pb), and adsorbing species with multiple bonds (e.g. CO, unsaturated hydrocarbons). For example, sulfur disrupts the production of methanol by poisoning the Cu/ZnO catalyst. Substances that increase reaction rate are called promoters. For example, the presence of alkali metals in ammonia synthesis increases the rate of N2 dissociation. The presence of poisons and promoters can alter theactivation energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules p ...
of the rate-limiting step and affect a catalyst's selectivity for the formation of certain products. Depending on the amount, a substance can be favorable or unfavorable for a chemical process. For example, in the production of ethylene, a small amount of chemisorbed chlorine will act as a promoter by improving Ag-catalyst selectivity towards ethylene over CO2, while too much chlorine will act as a poison.
Other mechanisms for catalyst deactivation include:
* Sintering
Clinker nodules produced by sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction.
Sintering happens as part of a manufacturing ...
: when heated, dispersed catalytic metal particles can migrate across the support surface and form crystals. This results in a reduction of catalyst surface area.
* Fouling
Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms ( biofouling) or a non-living substance (inorganic or organic). Fouling is usually distinguished from other sur ...
: the deposition of materials from the fluid phase onto the solid phase catalyst and/or support surfaces. This results in active site and/or pore blockage.
* Coking: the deposition of heavy, carbon-rich solids onto surfaces due to the decomposition of hydrocarbons
* Vapor-solid reactions: formation of an inactive surface layer and/or formation of a volatile compound that exits the reactor. This results in a loss of surface area and/or catalyst material.
* Solid-state transformation: solid-state diffusion of catalyst support atoms to the surface followed by a reaction that forms an inactive phase. This results in a loss of catalyst surface area.
* Erosion: continual attrition of catalyst material common in fluidized-bed reactors. This results in a loss of catalyst material.
In industry, catalyst deactivation costs billions every year due to process shutdown and catalyst replacement.
Industrial examples
In industry, many design variables must be considered including reactor and catalyst design across multiple scales ranging from the subnanometer to tens of meters. The conventional heterogeneous catalysis reactors includebatch
Batch may refer to:
Food and drink
* Batch (alcohol), an alcoholic fruit beverage
* Batch loaf, a type of bread popular in Ireland
* A dialect term for a bread roll used in North Warwickshire, Nuneaton and Coventry, as well as on the Wirra ...
, continuous
Continuity or continuous may refer to:
Mathematics
* Continuity (mathematics), the opposing concept to discreteness; common examples include
** Continuous probability distribution or random variable in probability and statistics
** Continuous ...
, and fluidized-bed reactors, while more recent setups include fixed-bed, microchannel, and multi-functional reactors. Other variables to consider are reactor dimensions, surface area, catalyst type, catalyst support, as well as reactor operating conditions such as temperature, pressure, and reactant concentrations.
 Some large-scale industrial processes incorporating heterogeneous catalysts are listed below.
Some large-scale industrial processes incorporating heterogeneous catalysts are listed below.
Other examples
*Reduction of nitriles in the synthesis of phenethylamine with Raney nickel catalyst and hydrogen inammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wa ...
:
* The cracking, isomerisation, and reformation of hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or e ...
to form appropriate and useful blends of petrol.
*In automobiles, catalytic converter
A catalytic converter is an vehicle emissions control, exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalysis, catalyzing a redox chemic ...
s are used to catalyze three main reactions:
**The oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
to carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
:
**:2CO(g) + O2(g) → 2CO2(g)
**The reduction of nitrogen monoxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its c ...
back to nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
:
**:2NO(g) + 2CO(g) → N2(g) + 2CO2(g)
**The oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
of hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or e ...
to water and carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is trans ...
:
**:2 C6H6 + 15 O2 → 12 CO2 + 6 H2O
*This process can occur with any of hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
, but most commonly is performed with petrol
Gasoline (; ) or petrol (; ) (see ) is a transparent, petroleum-derived flammable liquid that is used primarily as a fuel in most spark-ignited internal combustion engines (also known as petrol engines). It consists mostly of organic c ...
or diesel
Diesel may refer to:
* Diesel engine, an internal combustion engine where ignition is caused by compression
* Diesel fuel, a liquid fuel used in diesel engines
* Diesel locomotive, a railway locomotive in which the prime mover is a diesel engin ...
.
*Asymmetric heterogeneous catalysis facilitates the production of pure enantiomer compounds using chiral heterogeneous catalysts.
*The vast majority of heterogeneous catalysts are based on metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
s or metal oxides; however, some chemical reactions can be catalyzed by carbon-based materials, e.g., oxidative dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. At ...
s or selective oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
s.
**Ethylbenzene
Ethylbenzene is an organic compound with the formula . It is a highly flammable, colorless liquid with an odor similar to that of gasoline. This monocyclic aromatic hydrocarbon is important in the petrochemical industry as an reaction intermedia ...
+ 1/2 O2 → Styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
+ H2O
** Acrolein + 1/2 O2 → Acrylic acid
Acrylic acid (IUPAC: propenoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a ...
Solid-Liquid and Liquid-Liquid Catalyzed Reactions
Although the majority of heterogeneous catalysts are solids, there are a few variations which are of practical value. For two immiscible solutions (liquids), one carries the catalyst while the other carries the reactant. This set up is the basis of biphasic catalysis as implemented in the industrial production of butyraldehyde by the hydroformylation of propylene.See also
* Heterogeneous gold catalysis * Nanomaterial-based catalysts * Platinum nanoparticles * Temperature-programmed reduction * Thermal desorption spectroscopyReferences
External links
* {{Authority control Catalysis