
In
quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of
mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physical quantities of a
particle, such as
position
Position often refers to:
* Position (geometry), the spatial location (rather than orientation) of an entity
* Position, a job or occupation
Position may also refer to:
Games and recreation
* Position (poker), location relative to the dealer
* ...
, ''x'', and
momentum
In Newtonian mechanics, momentum (more specifically linear momentum or translational momentum) is the product of the mass and velocity of an object. It is a vector quantity, possessing a magnitude and a direction. If is an object's mass an ...
, ''p'', can be predicted from
initial condition
In mathematics and particularly in dynamic systems, an initial condition, in some contexts called a seed value, is a value of an evolving variable at some point in time designated as the initial time (typically denoted ''t'' = 0). For ...
s.
Such variable pairs are known as
complementary variables or
canonically conjugate variables; and, depending on interpretation, the uncertainty principle limits to what extent such conjugate properties maintain their approximate meaning, as the mathematical framework of
quantum physics
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
does not support the notion of simultaneously well-defined conjugate properties expressed by a single value. The uncertainty principle implies that it is in general not possible to predict the value of a quantity with arbitrary certainty, even if all initial conditions are specified.
Introduced first in 1927 by the German physicist
Werner Heisenberg, the uncertainty principle states that the more precisely the position of some particle is determined, the less precisely its momentum can be predicted from initial conditions, and vice versa. In the published 1927 paper, Heisenberg originally concluded that the uncertainty principle was
p
q ~ h using the full Planck constant.
[.
Annotated pre-publication proof sheet o]
Über den anschaulichen Inhalt der quantentheoretischen Kinematik und Mechanik
March 21, 1927. The formal inequality relating the
standard deviation
In statistics, the standard deviation is a measure of the amount of variation or dispersion of a set of values. A low standard deviation indicates that the values tend to be close to the mean (also called the expected value) of the set, while ...
of position ''σ
x'' and the standard deviation of momentum ''σ
p'' was derived by
Earle Hesse Kennard later that year and by
Hermann Weyl
Hermann Klaus Hugo Weyl, (; 9 November 1885 – 8 December 1955) was a German mathematician, theoretical physicist and philosopher. Although much of his working life was spent in Zürich, Switzerland, and then Princeton, New Jersey, he is assoc ...
in 1928:
where is the
reduced Planck constant, ).
Historically, the uncertainty principle has been confused
with a related effect in
physics, called the
observer effect, which notes that measurements of certain systems cannot be made without affecting the system, that is, without changing something in a system. Heisenberg utilized such an observer effect at the quantum level (see below) as a physical "explanation" of quantum uncertainty. It has since become clearer, however, that the uncertainty principle is inherent in the properties of all
wave-like systems,
and that it arises in quantum mechanics simply due to the
matter wave nature of all quantum objects. Thus, ''the uncertainty principle actually states a fundamental property of quantum systems and is not a statement about the observational success of current technology''.
Indeed the uncertainty principle has its roots in how we apply calculus to write the basic equations of mechanics. It must be emphasized that ''measurement'' does not mean only a process in which a physicist-observer takes part, but rather any interaction between classical and quantum objects regardless of any observer.
Since the uncertainty principle is such a basic result in quantum mechanics, typical experiments in quantum mechanics routinely observe aspects of it. Certain experiments, however, may deliberately test a particular form of the uncertainty principle as part of their main research program. These include, for example, tests of number–phase uncertainty relations in
superconducting or
quantum optics systems. Applications dependent on the uncertainty principle for their operation include extremely low-noise technology such as that required in
gravitational wave interferometers.
Introduction
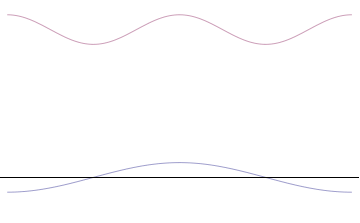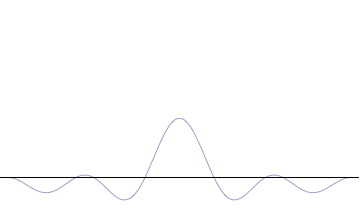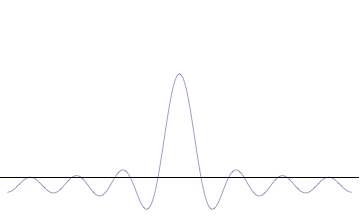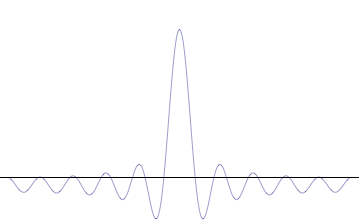
It is vital to illustrate how the principle applies to relatively intelligible physical situations since it is indiscernible on the macroscopic scales that humans experience. Two alternative frameworks for quantum physics offer different explanations for the uncertainty principle. The
wave mechanics picture of the uncertainty principle is more visually intuitive, but the more abstract
matrix mechanics picture formulates it in a way that generalizes more easily.
Mathematically, in wave mechanics, the uncertainty relation between position and momentum arises because the expressions of the wavefunction in the two corresponding
orthonormal bases in
Hilbert space
In mathematics, Hilbert spaces (named after David Hilbert) allow generalizing the methods of linear algebra and calculus from (finite-dimensional) Euclidean vector spaces to spaces that may be infinite-dimensional. Hilbert spaces arise natural ...
are
Fourier transforms
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
of one another (i.e., position and momentum are
conjugate variables
Conjugate variables are pairs of variables mathematically defined in such a way that they become Fourier transform duals, or more generally are related through Pontryagin duality. The duality relations lead naturally to an uncertainty relation— ...
). A nonzero function and its Fourier transform cannot both be sharply localized at the same time. A similar tradeoff between the variances of Fourier conjugates arises in all systems underlain by Fourier analysis, for example in sound waves: A pure tone is a
sharp spike at a single frequency, while its Fourier transform gives the shape of the sound wave in the time domain, which is a completely delocalized sine wave. In quantum mechanics, the two key points are that the position of the particle takes the form of a
matter wave, and momentum is its Fourier conjugate, assured by the
de Broglie relation , where is the
wavenumber.
In
matrix mechanics, the
mathematical formulation of quantum mechanics, any pair of non-
commuting self-adjoint operators representing
observables are subject to similar uncertainty limits. An eigenstate of an observable represents the state of the wavefunction for a certain measurement value (the eigenvalue). For example, if a measurement of an observable is performed, then the system is in a particular eigenstate of that observable. However, the particular eigenstate of the observable need not be an eigenstate of another observable : If so, then it does not have a unique associated measurement for it, as the system is not in an eigenstate of that observable.
Wave mechanics interpretation
(Ref
[Online copy]
)
According to the
de Broglie hypothesis
Matter waves are a central part of the theory of quantum mechanics, being an example of wave–particle duality. All matter exhibits wave-like behavior. For example, a beam of electrons can be diffracted just like a beam of light or a water wave ...
, every object in the universe is a
wave, i.e., a situation which gives rise to this phenomenon. The position of the particle is described by a
wave function . The time-independent wave function of a single-moded plane wave of wavenumber ''k''
0 or momentum ''p''
0 is
The
Born rule states that this should be interpreted as a
probability density amplitude function in the sense that the probability of finding the particle between ''a'' and ''b'' is
In the case of the single-moded plane wave,
is a
uniform distribution
Uniform distribution may refer to:
* Continuous uniform distribution
* Discrete uniform distribution
* Uniform distribution (ecology)
* Equidistributed sequence In mathematics, a sequence (''s''1, ''s''2, ''s''3, ...) of real numbers is said to be ...
. In other words, the particle position is extremely uncertain in the sense that it could be essentially anywhere along the wave packet.
On the other hand, consider a wave function that is a
sum of many waves, which we may write as
where ''A''
''n'' represents the relative contribution of the mode ''p''
''n'' to the overall total. The figures to the right show how with the addition of many plane waves, the wave packet can become more localized. We may take this a step further to the
continuum limit, where the wave function is an
integral over all possible modes
with
representing the amplitude of these modes and is called the wave function in
momentum space. In mathematical terms, we say that
is the ''
Fourier transform
A Fourier transform (FT) is a mathematical transform that decomposes functions into frequency components, which are represented by the output of the transform as a function of frequency. Most commonly functions of time or space are transformed, ...
'' of
and that ''x'' and ''p'' are
conjugate variables
Conjugate variables are pairs of variables mathematically defined in such a way that they become Fourier transform duals, or more generally are related through Pontryagin duality. The duality relations lead naturally to an uncertainty relation— ...
. Adding together all of these plane waves comes at a cost, namely the momentum has become less precise, having become a mixture of waves of many different momenta.
One way to quantify the precision of the position and momentum is the
standard deviation
In statistics, the standard deviation is a measure of the amount of variation or dispersion of a set of values. A low standard deviation indicates that the values tend to be close to the mean (also called the expected value) of the set, while ...
''σ''. Since
is a probability density function for position, we calculate its standard deviation.
The precision of the position is improved, i.e. reduced ''σ''
''x'', by using many plane waves, thereby weakening the precision of the momentum, i.e. increased ''σ''
''p''. Another way of stating this is that ''σ''
''x'' and ''σ''
''p'' have an
inverse relationship or are at least bounded from below. This is the uncertainty principle, the exact limit of which is the Kennard bound. Click the ''show'' button below to see a semi-formal derivation of the Kennard inequality using wave mechanics.
Matrix mechanics interpretation
(Ref
)
In matrix mechanics, observables such as position and momentum are represented by
self-adjoint operators. When considering pairs of observables, an important quantity is the ''
commutator
In mathematics, the commutator gives an indication of the extent to which a certain binary operation fails to be commutative. There are different definitions used in group theory and ring theory.
Group theory
The commutator of two elements, a ...
''. For a pair of operators and
, one defines their commutator as
In the case of position and momentum, the commutator is the
canonical commutation relation
The physical meaning of the non-commutativity can be understood by considering the effect of the commutator on position and momentum
eigenstate
In quantum physics, a quantum state is a mathematical entity that provides a probability distribution for the outcomes of each possible measurement on a system. Knowledge of the quantum state together with the rules for the system's evolution in t ...
s. Let
be a right eigenstate of position with a constant eigenvalue . By definition, this means that
Applying the commutator to
yields
where is the
identity operator.
Suppose, for the sake of
proof by contradiction, that
is also a right eigenstate of momentum, with constant eigenvalue . If this were true, then one could write
On the other hand, the above canonical commutation relation requires that
This implies that no quantum state can simultaneously be both a position and a momentum eigenstate.
When a state is measured, it is projected onto an eigenstate in the basis of the relevant observable. For example, if a particle's position is measured, then the state amounts to a position eigenstate. This means that the state is ''not'' a momentum eigenstate, however, but rather it can be represented as a sum of multiple momentum basis eigenstates. In other words, the momentum must be less precise. This precision may be quantified by the
standard deviation
In statistics, the standard deviation is a measure of the amount of variation or dispersion of a set of values. A low standard deviation indicates that the values tend to be close to the mean (also called the expected value) of the set, while ...
s,
As in the wave mechanics interpretation above, one sees a tradeoff between the respective precisions of the two, quantified by the uncertainty principle.
Heisenberg limit
In
quantum metrology, and especially
interferometry
Interferometry is a technique which uses the ''interference'' of superimposed waves to extract information. Interferometry typically uses electromagnetic waves and is an important investigative technique in the fields of astronomy, fiber opt ...
, the Heisenberg limit is the optimal rate at which the accuracy of a measurement can scale with the energy used in the measurement. Typically, this is the measurement of a phase (applied to one arm of a
beam-splitter) and the energy is given by the number of photons used in an
interferometer
Interferometry is a technique which uses the ''interference'' of superimposed waves to extract information. Interferometry typically uses electromagnetic waves and is an important investigative technique in the fields of astronomy, fiber op ...
. Although some claim to have broken the Heisenberg limit, this reflects disagreement on the definition of the scaling resource. Suitably defined, the Heisenberg limit is a consequence of the basic principles of quantum mechanics and cannot be beaten, although the weak Heisenberg limit can be beaten.
Robertson–Schrödinger uncertainty relations
The most common general form of the uncertainty principle is the ''
Robertson uncertainty relation''.
For an arbitrary
Hermitian operator we can associate a standard deviation
where the brackets
indicate an
expectation value. For a pair of operators
and
, we may define their ''
commutator
In mathematics, the commutator gives an indication of the extent to which a certain binary operation fails to be commutative. There are different definitions used in group theory and ring theory.
Group theory
The commutator of two elements, a ...
'' as
In this notation, the Robertson uncertainty relation is given by
The Robertson uncertainty relation immediately
follows from
Follows is a surname. Notable people with the surname include:
* Dave Follows (1941–2003), British cartoonist
* Denis Follows (1908–1983), British sports administrator
* Geoffrey Follows (1896–1983), British colonial administrator
* Megan Fo ...
a slightly stronger inequality, the ''Schrödinger uncertainty relation'',
where we have introduced the ''anticommutator'',
Mixed states
The Robertson–Schrödinger uncertainty relation may be generalized in a straightforward way to describe
mixed states.
The Maccone–Pati uncertainty relations
The Robertson–Schrödinger uncertainty relation can be trivial if the state of the system is chosen to be eigenstate of one of the observable. The stronger uncertainty relations proved by Maccone and Pati give non-trivial bounds on the sum of the variances for two incompatible observables. (Earlier works on uncertainty relations formulated as the sum of variances include, e.g., Ref. due to Huang.) For two non-commuting observables
and
the first stronger uncertainty relation is given by
where
,
,
is a normalized vector that is orthogonal to the state of the system
and one should choose the sign of
to make this real quantity a positive number.
The second stronger uncertainty relation is given by
where
is a state orthogonal to
.
The form of
implies that the right-hand side of the new uncertainty relation is nonzero unless
is an eigenstate of
. One may note that
can be an eigenstate of
without being an eigenstate of either
or
. However, when
is an eigenstate of one of the two observables the Heisenberg–Schrödinger uncertainty relation becomes trivial. But the lower bound in the new relation is nonzero unless
is an eigenstate of both.
Improving the Robertson–Schrödinger uncertainty relation based on decompositions of the density matrix
The Robertson–Schrödinger uncertainty can be improved noting that it must hold for all components
in any decomposition of the density matrix given as
Here, for the probailities
and
hold. Then, using the relation
for
,
it follows that
where the function in the bound is defined
The above relation very often has a bound larger than that of the original Robertson–Schrödinger uncertainty relation. Thus, we need to calculate the bound of the Robertson–Schrödinger uncertainty for the mixed components of the quantum state rather than for the quantum state, and compute an average of their square roots. The following expression is stronger than the Robertson–Schrödinger uncertainty relation
where on the right-hand side there is a concave roof over the decompositions of the density matrix.
The improved relation above is saturated by all single-qubit quantum states.
With similar arguments, one can derive a relation with a convex roof on the right-hand side
where
 In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physical quantities of a particle, such as
In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physical quantities of a particle, such as  It is vital to illustrate how the principle applies to relatively intelligible physical situations since it is indiscernible on the macroscopic scales that humans experience. Two alternative frameworks for quantum physics offer different explanations for the uncertainty principle. The wave mechanics picture of the uncertainty principle is more visually intuitive, but the more abstract matrix mechanics picture formulates it in a way that generalizes more easily.
Mathematically, in wave mechanics, the uncertainty relation between position and momentum arises because the expressions of the wavefunction in the two corresponding orthonormal bases in
It is vital to illustrate how the principle applies to relatively intelligible physical situations since it is indiscernible on the macroscopic scales that humans experience. Two alternative frameworks for quantum physics offer different explanations for the uncertainty principle. The wave mechanics picture of the uncertainty principle is more visually intuitive, but the more abstract matrix mechanics picture formulates it in a way that generalizes more easily.
Mathematically, in wave mechanics, the uncertainty relation between position and momentum arises because the expressions of the wavefunction in the two corresponding orthonormal bases in  In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physical quantities of a particle, such as
In quantum mechanics, the uncertainty principle (also known as Heisenberg's uncertainty principle) is any of a variety of mathematical inequalities asserting a fundamental limit to the accuracy with which the values for certain pairs of physical quantities of a particle, such as  It is vital to illustrate how the principle applies to relatively intelligible physical situations since it is indiscernible on the macroscopic scales that humans experience. Two alternative frameworks for quantum physics offer different explanations for the uncertainty principle. The wave mechanics picture of the uncertainty principle is more visually intuitive, but the more abstract matrix mechanics picture formulates it in a way that generalizes more easily.
Mathematically, in wave mechanics, the uncertainty relation between position and momentum arises because the expressions of the wavefunction in the two corresponding orthonormal bases in
It is vital to illustrate how the principle applies to relatively intelligible physical situations since it is indiscernible on the macroscopic scales that humans experience. Two alternative frameworks for quantum physics offer different explanations for the uncertainty principle. The wave mechanics picture of the uncertainty principle is more visually intuitive, but the more abstract matrix mechanics picture formulates it in a way that generalizes more easily.
Mathematically, in wave mechanics, the uncertainty relation between position and momentum arises because the expressions of the wavefunction in the two corresponding orthonormal bases in