Harries Ozonide Reaction on:
[Wikipedia]
[Google]
[Amazon]
In
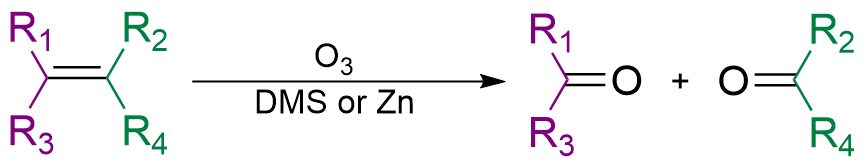 An example is the ozonolysis of
An example is the ozonolysis of  By controlling the reaction/workup conditions, unsymmetrical products can be generated from symmetrical alkenes:
* Using TsOH;
By controlling the reaction/workup conditions, unsymmetrical products can be generated from symmetrical alkenes:
* Using TsOH;
 In the generally accepted mechanism proposed by
In the generally accepted mechanism proposed by  Evidence for this mechanism is found in isotopic labeling. When 17O-labelled
Evidence for this mechanism is found in isotopic labeling. When 17O-labelled

\underset + 4O3 -> \underset + \underset
 The method was used to confirm the structural repeat unit in natural rubber as
The method was used to confirm the structural repeat unit in natural rubber as
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, ozonolysis is an organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, Mechanistic Organ ...
where the unsaturated bonds of alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s (), alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s (), or azo compounds () are cleaved with ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
(). Alkenes and alkynes form organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s in which the multiple carbon–carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is formed b ...
has been replaced by a carbonyl () group while azo compounds form nitrosamine
In organic chemistry, nitrosamines (or more formally ''N''-Nitrosamines) are organic compounds with the chemical structure , where R is usually an alkyl group. They feature a nitroso group () bonded to a deprotonated amine. Most nitrosamines are ...
s (). The outcome of the reaction depends on the type of multiple bond being oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
and the work-up conditions.
Ozonolysis of alkenes
Alkenes can be oxidized withozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
to form alcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
s, aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
s or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s, or carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates complete consumption of the alkene. Alternatively, various other chemicals can be used as indicators of this endpoint by detecting the presence of ozone. If ozonolysis is performed by bubbling a stream of ozone-enriched oxygen through the reaction mixture, the gas that bubbles out can be directed through a potassium iodide solution. When the solution has stopped absorbing ozone, the ozone in the bubbles oxidizes the iodide to iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
, which can easily be observed by its violet color. For closer control of the reaction itself, an indicator such as Sudan Red III can be added to the reaction mixture. Ozone reacts with this indicator more slowly than with the intended ozonolysis target. The ozonolysis of the indicator, which causes a noticeable color change, only occurs once the desired target has been consumed. If the substrate has two alkenes that react with ozone at different rates, one can choose an indicator whose own oxidation rate is intermediate between them, and therefore stop the reaction when only the most susceptible alkene in the substrate has reacted. Otherwise, the presence of unreacted ozone in solution (seeing its blue color) or in the bubbles (via iodide detection) only indicates when all alkenes have reacted.
After completing the addition, a reagent is then added to convert the intermediate ozonide to a carbonyl derivative. Reductive work-up conditions are far more commonly used than oxidative conditions. The use of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists a ...
, thiourea, zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
dust, or dimethyl sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cook ...
produces aldehydes or ketones while the use of sodium borohydride produces alcohols. The use of hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
produces carboxylic acids. Recently, the use of amine ''N''-oxides has been reported to produce aldehydes directly. Other functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s, such as benzyl ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
s, can also be oxidized by ozone. It has been proposed that small amounts of acid may be generated during the reaction from oxidation of the solvent, so pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
is sometimes used to buffer
Buffer may refer to:
Science
* Buffer gas, an inert or nonflammable gas
* Buffer solution, a solution used to prevent changes in pH
* Buffering agent, the weak acid or base in a buffer solution
* Lysis buffer, in cell biology
* Metal ion buffer
* ...
the reaction. Dichloromethane
Dichloromethane (DCM or methylene chloride, methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odour is widely used as a solvent. Although it is not miscible with ...
is often used as a 1:1 cosolvent to facilitate timely cleavage of the ozonide. Azelaic acid
Azelaic acid (AzA) is an organic compound with the formula HOOC(CH2)7 COOH. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a precursor to diverse industrial products including polymers an ...
and pelargonic acids are produced from ozonolysis of oleic acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega ...
on an industrial scale.
: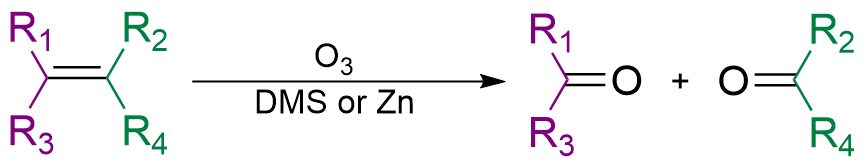 An example is the ozonolysis of
An example is the ozonolysis of eugenol
Eugenol is an allyl chain-substituted guaiacol, a member of the allylbenzene class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove, nutmeg, cinnamon, basil ...
converting the terminal alkene to an aldehyde:
: By controlling the reaction/workup conditions, unsymmetrical products can be generated from symmetrical alkenes:
* Using TsOH;
By controlling the reaction/workup conditions, unsymmetrical products can be generated from symmetrical alkenes:
* Using TsOH; sodium bicarbonate
Sodium bicarbonate (IUPAC name: sodium hydrogencarbonate), commonly known as baking soda or bicarbonate of soda, is a chemical compound with the formula NaHCO3. It is a salt composed of a sodium cation ( Na+) and a bicarbonate anion ( HCO3−) ...
(NaHCO3); dimethyl sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cook ...
(DMS) gives an aldehyde and a dimethyl acetal
* Using acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a col ...
(Ac2O), triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
(Et3N) gives a methyl ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
and an aldehyde
* Using TsOH; Ac2O, Et3N, gives a methyl ester and a dimethyl acetal.
Reaction mechanism
Rudolf Criegee
Rudolf Criegee (* May 23, 1902 in Düsseldorf; † November 7, 1975 in Karlsruhe) was a German organic chemist.
Early life
Criegee's family was wealthy. His father worked as a court director. The family was national liberal, Prussian and Prot ...
in 1953, the alkene and ozone form an intermediate molozonide
A molozonide (or "molecular ozonide") is a 1,2,3-trioxolane, which can also be thought of a cyclic dialkyl trioxidane. Molozonides are formed by cycloaddition of ozone and an alkene during ozonolysis, as a transient intermediate which quickly rearr ...
in a 1,3-dipolar cycloaddition. Next, the molozonide reverts to its corresponding carbonyl oxide (also called the Criegee intermediate or Criegee zwitterion) and aldehyde or ketone in a retro-1,3-dipolar cycloaddition. The oxide and aldehyde or ketone react again in a 1,3-dipolar cycloaddition or produce a relatively stable ozonide intermediate (a trioxolane).
: Evidence for this mechanism is found in isotopic labeling. When 17O-labelled
Evidence for this mechanism is found in isotopic labeling. When 17O-labelled benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
reacts with carbonyl oxides, the label ends up exclusively in the ether linkage of the ozonide. There is still dispute over whether the molozonide collapses via a concerted or radical process; this may also exhibit a substrate dependence.
History
Christian Friedrich Schönbein
Christian Friedrich Schönbein H FRSE(18 October 1799 – 29 August 1868) was a German-Swiss chemist who is best known for inventing the fuel cell (1838) at the same time as William Robert Grove and his discoveries of guncotton and ozone.
Lif ...
, who discovered ozone in 1840, also did the first ozonolysis: in 1845, he reported that ethylene reacts with ozone – after the reaction, neither the smell of ozone nor the smell of ethylene was perceivable.
The ozonolysis of alkenes is sometimes referred to as "Harries ozonolysis", because some attribute this reaction to Carl Dietrich Harries
Carl Dietrich Harries (5 August 1866 – 3 November 1923) was a German chemist born in Luckenwalde, Brandenburg, Prussia. He received his doctorate in 1892. In 1900, he married Hertha von Siemens, daughter of the electrical genius Werner von Sie ...
.
Before the advent of modern spectroscopic techniques, the ozonolysis was an important method for determining the structure of organic molecules. Chemists would ozonize an unknown alkene to yield smaller and more readily identifiable fragments.
Ozonolysis of alkynes
Ozonolysis ofalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s generally gives an acid anhydride or diketone
In organic chemistry, a dicarbonyl is a molecule containing two carbonyl () groups. Although this term could refer to any organic compound containing two carbonyl groups, it is used more specifically to describe molecules in which both carbonyls ...
product, not complete fragmentation as for alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s. A reducing agent is not needed for these reactions. The exact mechanism is not completely known. If the reaction is performed in the presence of water, the anhydride hydrolyzes to give two carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s.
:
Ozonolysis of other substrates
Ozonolysis ofoleic acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega ...
is an important route to azelaic acid
Azelaic acid (AzA) is an organic compound with the formula HOOC(CH2)7 COOH. This saturated dicarboxylic acid exists as a white powder. It is found in wheat, rye, and barley. It is a precursor to diverse industrial products including polymers an ...
. The coproduct is nonanoic acid
Pelargonic acid, also called nonanoic acid, is an organic compound with structural formula CH3(CH2)7CO2H. It is a nine-carbon fatty acid. Nonanoic acid is a colorless oily liquid with an unpleasant, rancid odor. It is nearly insoluble in water, ...
:
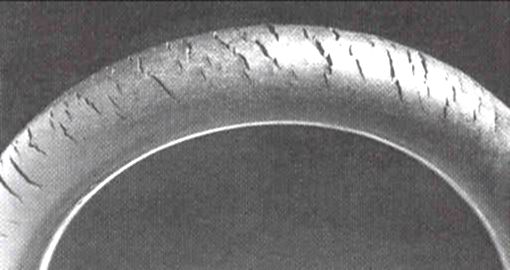
:Ozonolysis of elastomers
 The method was used to confirm the structural repeat unit in natural rubber as
The method was used to confirm the structural repeat unit in natural rubber as isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. Isoprene is an unsaturated hydrocarbon. It is produced by many plants and animals ...
. It is also a serious problem, known as ozone cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those ...
where traces of the gas in an atmosphere cause degradation in elastomers, such as natural rubber, polybutadiene
Polybutadiene utadiene rubber BRis a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tir ...
, styrene-butadiene and nitrile rubber
Nitrile rubber, also known as nitrile butadiene rubber, NBR, Buna-N, and acrylonitrile butadiene rubber, is a synthetic rubber derived from acrylonitrile (ACN) and butadiene. Trade names include Perbunan, Nipol, Krynac and Europrene. This rubber is ...
. Ozonolysis creates surface ketone groups which can cause further gradual degradation via Norrish reaction A Norrish reaction in organic chemistry is a photochemical reaction taking place with ketones and aldehydes. Such reactions are subdivided into Norrish type I reactions and Norrish type II reactions. The reaction is named after Ronald George Wreyfo ...
s if the polymer is exposed to light.
Ozone cracking is a form of stress corrosion cracking
Stress corrosion cracking (SCC) is the growth of crack formation in a corrosive environment. It can lead to unexpected and sudden failure of normally ductile metal alloys subjected to a tensile stress, especially at elevated temperature. SCC ...
where active chemical species attack products of a susceptible material. The rubber product must be under tension for crack growth to occur. Ozone cracking was once commonly seen in the sidewalls of tire
A tire (American English) or tyre (British English) is a ring-shaped component that surrounds a Rim (wheel), wheel's rim to transfer a vehicle's load from the axle through the wheel to the ground and to provide Traction (engineering), t ...
s, where it could expand to cause a dangerous blowout
Blowout or Blow out may refer to:
Film and television
*''Blow Out'', a 1981 film by Brian De Palma
* ''The Blow Out'', a 1936 short film
* ''Blow Out'' (TV series), a TV series on Bravo
* "Blow Out" (''Prison Break''), an episode of ''Prison ...
, but is now rare owing to the use of modern antiozonants. Other means of prevention include replacing susceptible rubbers with resistant elastomers such as polychloroprene
Neoprene (also polychloroprene) is a family of synthetic rubbers that are produced by polymerization of chloroprene.Werner Obrecht, Jean-Pierre Lambert, Michael Happ, Christiane Oppenheimer-Stix, John Dunn and Ralf Krüger "Rubber, 4. Emulsion R ...
, EPDM or Viton.
See also
* Polymer degradation *Lemieux–Johnson oxidation The Lemieux–Johnson or Malaprade–Lemieux–Johnson oxidation is a chemical reaction in which an olefin undergoes oxidative cleavage to form two aldehyde or ketone units. The reaction is named after its inventors, Raymond Urgel Lemieux and Will ...
– an alternative system using periodate and osmium tetroxide
*'' Trametes hirsuta'', a biotechnological
Biotechnology is the integration of natural sciences and engineering sciences in order to achieve the application of organisms, cells, parts thereof and molecular analogues for products and services. The term ''biotechnology'' was first used by ...
alternative to ozonolysis.
References
{{Organic reactions Organic oxidation reactions Cycloadditions