Hafnium Diselenide on:
[Wikipedia]
[Google]
[Amazon]
Hafnium is a chemical element with the
 Hafnium is a shiny, silvery, ductile metal that is corrosion-resistant and chemically similar to zirconium (due to its having the same number of valence electrons, being in the same group, but also to relativistic effects; the expected expansion of atomic radii from period 5 to 6 is almost exactly cancelled out by the lanthanide contraction). Hafnium changes from its alpha form, a hexagonal close-packed lattice, to its beta form, a body-centered cubic lattice, at 2388 K. The physical properties of hafnium metal samples are markedly affected by zirconium impurities, especially the nuclear properties, as these two elements are among the most difficult to separate because of their chemical similarity.
A notable physical difference between these metals is their density, with zirconium having about one-half the density of hafnium. The most notable
Hafnium is a shiny, silvery, ductile metal that is corrosion-resistant and chemically similar to zirconium (due to its having the same number of valence electrons, being in the same group, but also to relativistic effects; the expected expansion of atomic radii from period 5 to 6 is almost exactly cancelled out by the lanthanide contraction). Hafnium changes from its alpha form, a hexagonal close-packed lattice, to its beta form, a body-centered cubic lattice, at 2388 K. The physical properties of hafnium metal samples are markedly affected by zirconium impurities, especially the nuclear properties, as these two elements are among the most difficult to separate because of their chemical similarity.
A notable physical difference between these metals is their density, with zirconium having about one-half the density of hafnium. The most notable
 Hafnium reacts in air to form a protective film that inhibits further corrosion. The metal is not readily attacked by acids but can be oxidized with
Hafnium reacts in air to form a protective film that inhibits further corrosion. The metal is not readily attacked by acids but can be oxidized with
 Hafnium is estimated to make up about 5.8 ppm of the Earth's upper crust by mass. It does not exist as a free element on Earth, but is found combined in solid solution with zirconium in natural zirconium compounds such as zircon, ZrSiO4, which usually has about 1–4% of the Zr replaced by Hf. Rarely, the Hf/Zr ratio increases during crystallization to give the isostructural mineral hafnon , with atomic Hf > Zr. An obsolete name for a variety of zircon containing unusually high Hf content is ''alvite''.
A major source of zircon (and hence hafnium) ores is heavy mineral sands ore deposits,
Hafnium is estimated to make up about 5.8 ppm of the Earth's upper crust by mass. It does not exist as a free element on Earth, but is found combined in solid solution with zirconium in natural zirconium compounds such as zircon, ZrSiO4, which usually has about 1–4% of the Zr replaced by Hf. Rarely, the Hf/Zr ratio increases during crystallization to give the isostructural mineral hafnon , with atomic Hf > Zr. An obsolete name for a variety of zircon containing unusually high Hf content is ''alvite''.
A major source of zircon (and hence hafnium) ores is heavy mineral sands ore deposits,
 The heavy mineral sands ore deposits of the titanium ores ilmenite and rutile yield most of the mined zirconium, and therefore also most of the hafnium.
Zirconium is a good nuclear fuel-rod cladding metal, with the desirable properties of a very low neutron capture cross section and good chemical stability at high temperatures. However, because of hafnium's neutron-absorbing properties, hafnium impurities in zirconium would cause it to be far less useful for nuclear-reactor applications. Thus, a nearly complete separation of zirconium and hafnium is necessary for their use in nuclear power. The production of hafnium-free zirconium is the main source for hafnium.
The heavy mineral sands ore deposits of the titanium ores ilmenite and rutile yield most of the mined zirconium, and therefore also most of the hafnium.
Zirconium is a good nuclear fuel-rod cladding metal, with the desirable properties of a very low neutron capture cross section and good chemical stability at high temperatures. However, because of hafnium's neutron-absorbing properties, hafnium impurities in zirconium would cause it to be far less useful for nuclear-reactor applications. Thus, a nearly complete separation of zirconium and hafnium is necessary for their use in nuclear power. The production of hafnium-free zirconium is the main source for hafnium.
 The chemical properties of hafnium and zirconium are nearly identical, which makes the two difficult to separate. The methods first used —
The chemical properties of hafnium and zirconium are nearly identical, which makes the two difficult to separate. The methods first used — HfCl4 + 2Mg ->
Further purification is effected by a chemical transport reaction developed by Arkel and de Boer: In a closed vessel, hafnium reacts with Hf + 2I2 -> 00^oCHfI4
::HfI4 ->
 In his report on ''The Periodic Law of the Chemical Elements'', in 1869,
In his report on ''The Periodic Law of the Chemical Elements'', in 1869,
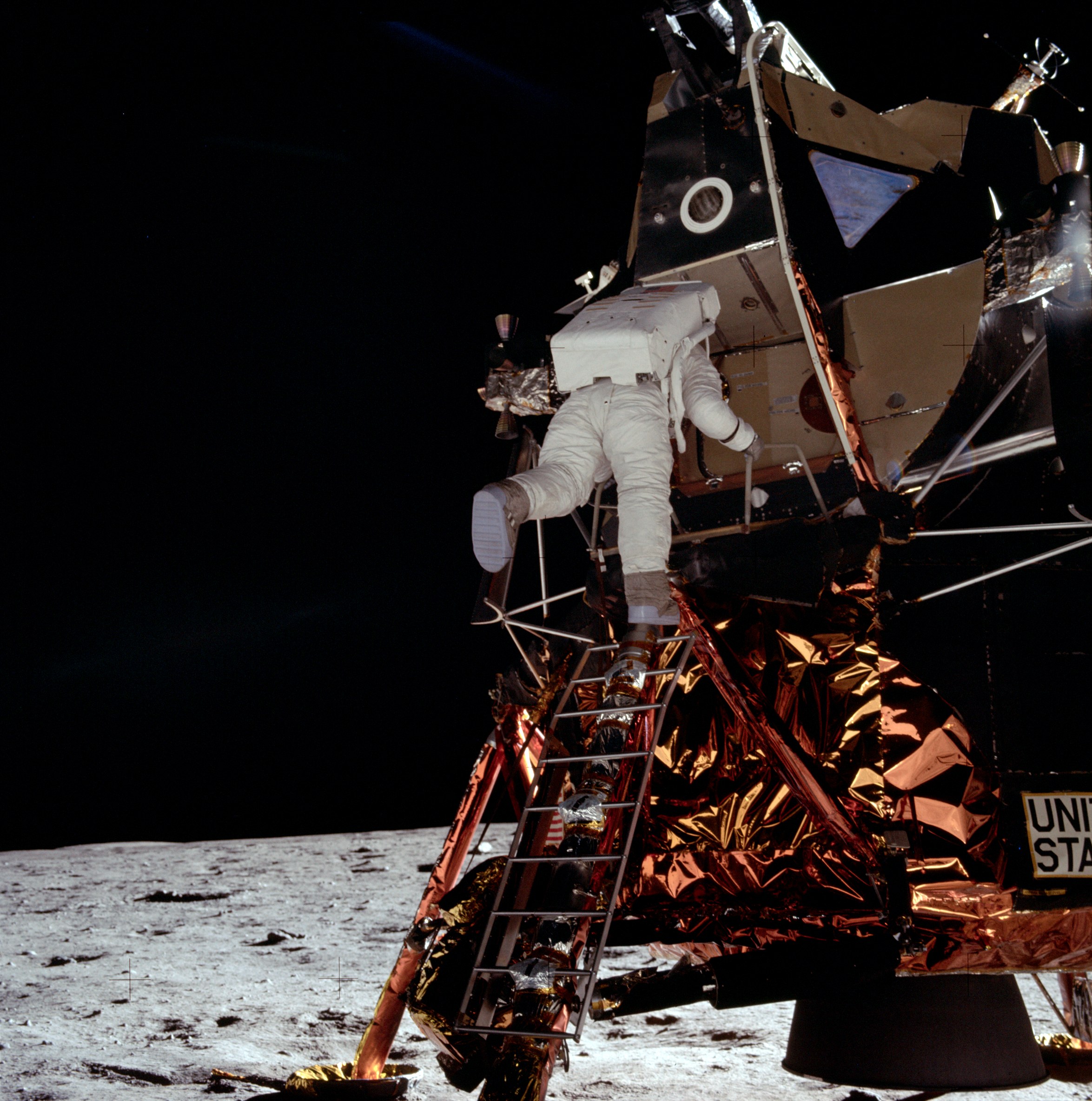 Hafnium is used in alloys with iron, titanium,
Hafnium is used in alloys with iron, titanium,
Hafnium
at Los Alamos National Laboratory'
periodic table of the elements
at '' The Periodic Table of Videos'' (University of Nottingham)
Hafnium Technical & Safety Data
NLM Hazardous Substances Databank – Hafnium, elemental
* Don Clark
Intel Shifts from Silicon to Lift Chip Performance
- WSJ, 2007
* ttps://www.cdc.gov/niosh/npg/npgd0309.html CDC - NIOSH Pocket Guide to Chemical Hazards* https://colnect.com/en/coins/list/composition/168-Hafnium {{Good article Chemical elements Transition metals Neutron poisons 1923 in science Chemical elements with hexagonal close-packed structure
symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri Mendeleev in 1869, though it was not identified until 1923, by Dirk Coster and George de Hevesy, making it the penultimate stable
A stable is a building in which livestock, especially horses, are kept. It most commonly means a building that is divided into separate stalls for individual animals and livestock. There are many different types of stables in use today; the ...
element to be discovered (the last being rhenium in 1925). Hafnium is named after , the Latin name for Copenhagen, where it was discovered.
Hafnium is used in filaments and electrodes. Some semiconductor fabrication processes use its oxide for integrated circuit
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Large numbers of tiny ...
s at 45 nanometers and smaller feature lengths. Some superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Several key characteristics of a superalloy are excellent mechanical strength, resistance to thermal creep deformation, g ...
s used for special applications contain hafnium in combination with niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
, titanium, or tungsten.
Hafnium's large neutron capture cross section makes it a good material for neutron absorption in control rod
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing ...
s in nuclear power plant
A nuclear power plant (NPP) is a thermal power station in which the heat source is a nuclear reactor. As is typical of thermal power stations, heat is used to generate steam that drives a steam turbine connected to a electric generator, generato ...
s, but at the same time requires that it be removed from the neutron-transparent corrosion-resistant zirconium alloy
Zirconium alloys are solid solutions of zirconium or other metals, a common subgroup having the trade mark Zircaloy. Zirconium has very low absorption cross-section of thermal neutrons, high hardness, ductility and corrosion resistance. One o ...
s used in nuclear reactors.
Characteristics
Physical characteristics
 Hafnium is a shiny, silvery, ductile metal that is corrosion-resistant and chemically similar to zirconium (due to its having the same number of valence electrons, being in the same group, but also to relativistic effects; the expected expansion of atomic radii from period 5 to 6 is almost exactly cancelled out by the lanthanide contraction). Hafnium changes from its alpha form, a hexagonal close-packed lattice, to its beta form, a body-centered cubic lattice, at 2388 K. The physical properties of hafnium metal samples are markedly affected by zirconium impurities, especially the nuclear properties, as these two elements are among the most difficult to separate because of their chemical similarity.
A notable physical difference between these metals is their density, with zirconium having about one-half the density of hafnium. The most notable
Hafnium is a shiny, silvery, ductile metal that is corrosion-resistant and chemically similar to zirconium (due to its having the same number of valence electrons, being in the same group, but also to relativistic effects; the expected expansion of atomic radii from period 5 to 6 is almost exactly cancelled out by the lanthanide contraction). Hafnium changes from its alpha form, a hexagonal close-packed lattice, to its beta form, a body-centered cubic lattice, at 2388 K. The physical properties of hafnium metal samples are markedly affected by zirconium impurities, especially the nuclear properties, as these two elements are among the most difficult to separate because of their chemical similarity.
A notable physical difference between these metals is their density, with zirconium having about one-half the density of hafnium. The most notable nuclear
Nuclear may refer to:
Physics
Relating to the nucleus of the atom:
*Nuclear engineering
*Nuclear physics
*Nuclear power
*Nuclear reactor
*Nuclear weapon
*Nuclear medicine
*Radiation therapy
*Nuclear warfare
Mathematics
*Nuclear space
* Nuclear ...
properties of hafnium are its high thermal neutron capture cross section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of ...
and that the nuclei of several different hafnium isotopes readily absorb two or more neutrons apiece. In contrast with this, zirconium is practically transparent to thermal neutrons, and it is commonly used for the metal components of nuclear reactors – especially the cladding of their nuclear fuel rods.
Chemical characteristics
 Hafnium reacts in air to form a protective film that inhibits further corrosion. The metal is not readily attacked by acids but can be oxidized with
Hafnium reacts in air to form a protective film that inhibits further corrosion. The metal is not readily attacked by acids but can be oxidized with halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s or it can be burnt in air. Like its sister metal zirconium, finely divided hafnium can ignite spontaneously in air. The metal is resistant to concentrated alkali
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a ...
s.
As a consequence of lanthanide contraction, the chemistry of hafnium and zirconium is so similar that the two cannot be separated on the basis of differing chemical reactions. The melting points and boiling points of the compounds and the solubility in solvents are the major differences in the chemistry of these twin elements.
Isotopes
At least 36 isotopes of hafnium have been observed, ranging in mass number from 153 to 188. The five stable isotopes are in the range of 176 to 180. The radioactive isotopes' half-lives range from 400 ms for 153Hf to years for the most stable one, theprimordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before ...
174Hf.
The extinct radionuclide 182Hf has a half-life of , and is an important tracker isotope for the formation of planetary core
A planetary core consists of the innermost layers of a planet. Cores may be entirely solid or entirely liquid, or a mixture of solid and liquid layers as is the case in the Earth. In the Solar System, core sizes range from about 20% (the Moon ...
s. The nuclear isomer 178m2Hf was at the center of a controversy for several years regarding its potential use as a weapon.
Occurrence
 Hafnium is estimated to make up about 5.8 ppm of the Earth's upper crust by mass. It does not exist as a free element on Earth, but is found combined in solid solution with zirconium in natural zirconium compounds such as zircon, ZrSiO4, which usually has about 1–4% of the Zr replaced by Hf. Rarely, the Hf/Zr ratio increases during crystallization to give the isostructural mineral hafnon , with atomic Hf > Zr. An obsolete name for a variety of zircon containing unusually high Hf content is ''alvite''.
A major source of zircon (and hence hafnium) ores is heavy mineral sands ore deposits,
Hafnium is estimated to make up about 5.8 ppm of the Earth's upper crust by mass. It does not exist as a free element on Earth, but is found combined in solid solution with zirconium in natural zirconium compounds such as zircon, ZrSiO4, which usually has about 1–4% of the Zr replaced by Hf. Rarely, the Hf/Zr ratio increases during crystallization to give the isostructural mineral hafnon , with atomic Hf > Zr. An obsolete name for a variety of zircon containing unusually high Hf content is ''alvite''.
A major source of zircon (and hence hafnium) ores is heavy mineral sands ore deposits, pegmatite
A pegmatite is an igneous rock showing a very coarse texture, with large interlocking crystals usually greater in size than and sometimes greater than . Most pegmatites are composed of quartz, feldspar, and mica, having a similar silicic com ...
s, particularly in Brazil and Malawi, and carbonatite intrusions, particularly the Crown Polymetallic Deposit at Mount Weld, Western Australia. A potential source of hafnium is trachyte tuffs containing rare zircon-hafnium silicates eudialyte or armstrongite
Armstrongite (CaZr i6O15�3H2O) is a silicate mineral.
Discovery and occurrence
It was first described in 1973 from an occurrence at Dorozhnyi pegmatite, Khanbogd District, Ömnögovi Province, Mongolia. It was named for the American astronaut Ne ...
, at Dubbo
Dubbo () is a city in the Orana Region of New South Wales, Australia. It is the largest population centre in the Orana region, with a population of 43,516 at June 2021.
The city is located at the intersection of the Newell, Mitchell, and Gol ...
in New South Wales, Australia.
Production
 The heavy mineral sands ore deposits of the titanium ores ilmenite and rutile yield most of the mined zirconium, and therefore also most of the hafnium.
Zirconium is a good nuclear fuel-rod cladding metal, with the desirable properties of a very low neutron capture cross section and good chemical stability at high temperatures. However, because of hafnium's neutron-absorbing properties, hafnium impurities in zirconium would cause it to be far less useful for nuclear-reactor applications. Thus, a nearly complete separation of zirconium and hafnium is necessary for their use in nuclear power. The production of hafnium-free zirconium is the main source for hafnium.
The heavy mineral sands ore deposits of the titanium ores ilmenite and rutile yield most of the mined zirconium, and therefore also most of the hafnium.
Zirconium is a good nuclear fuel-rod cladding metal, with the desirable properties of a very low neutron capture cross section and good chemical stability at high temperatures. However, because of hafnium's neutron-absorbing properties, hafnium impurities in zirconium would cause it to be far less useful for nuclear-reactor applications. Thus, a nearly complete separation of zirconium and hafnium is necessary for their use in nuclear power. The production of hafnium-free zirconium is the main source for hafnium.
 The chemical properties of hafnium and zirconium are nearly identical, which makes the two difficult to separate. The methods first used —
The chemical properties of hafnium and zirconium are nearly identical, which makes the two difficult to separate. The methods first used — fractional crystallization Fractional crystallization may refer to:
* Fractional crystallization (chemistry), a process to separate different solutes from a solution
* Fractional crystallization (geology)
Fractional crystallization, or crystal fractionation, is one of the ...
of ammonium fluoride salts or the fractional distillation of the chloride — have not proven suitable for an industrial-scale production. After zirconium was chosen as material for nuclear reactor programs in the 1940s, a separation method had to be developed. Liquid–liquid extraction processes with a wide variety of solvents were developed and are still used for the production of hafnium. About half of all hafnium metal manufactured is produced as a by-product of zirconium refinement. The end product of the separation is hafnium(IV) chloride
Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a cata ...
. The purified hafnium(IV) chloride is converted to the metal by reduction with magnesium or sodium, as in the Kroll process.
::100^oC
1 (one, unit, unity) is a number representing a single or the only entity. 1 is also a numerical digit and represents a single unit of counting or measurement. For example, a line segment of ''unit length'' is a line segment of length 1. ...
Hf + 2MgCl2iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
at temperatures of , forming hafnium(IV) iodide; at a tungsten filament of the reverse reaction happens preferentially, and the chemically bound iodine and hafnium dissociate into the native elements. The hafnium forms a solid coating at the tungsten filament, and the iodine can react with additional hafnium, resulting in a steady iodine turnover and ensuring the chemical equilibrium remains in favor of hafnium production.
::700^oC
7 (seven) is the natural number following 6 and preceding 8. It is the only prime number preceding a cube.
As an early prime number in the series of positive integers, the number seven has greatly symbolic associations in religion, mythology, s ...
Hf + 2I2Chemical compounds
Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4.Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
s react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
, sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
, and silicon. Some compounds of hafnium in lower oxidation states are known.
Hafnium(IV) chloride
Hafnium(IV) chloride is the inorganic compound with the formula HfCl4. This colourless solid is the precursor to most hafnium organometallic compounds. It has a variety of highly specialized applications, mainly in materials science and as a cata ...
and hafnium(IV) iodide have some applications in the production and purification of hafnium metal. They are volatile solids with polymeric structures. These tetrachlorides are precursors to various organohafnium compounds such as hafnocene dichloride and tetrabenzylhafnium.
The white hafnium oxide (HfO2), with a melting point of 2,812 °C and a boiling point of roughly 5,100 °C, is very similar to zirconia, but slightly more basic. Hafnium carbide
Hafnium carbide ( Hf C) is a chemical compound of hafnium and carbon. Previously the material was estimated to have a melting point of about 3,900 °C. More recent tests have been able to conclusively prove that the substance has an even hig ...
is the most refractory binary compound known, with a melting point over 3,890 °C, and hafnium nitride is the most refractory of all known metal nitrides, with a melting point of 3,310 °C. This has led to proposals that hafnium or its carbides might be useful as construction materials that are subjected to very high temperatures. The mixed carbide tantalum hafnium carbide () possesses the highest melting point of any currently known compound, . Recent supercomputer simulations suggest a hafnium alloy with a melting point of 4,400 K.
History
 In his report on ''The Periodic Law of the Chemical Elements'', in 1869,
In his report on ''The Periodic Law of the Chemical Elements'', in 1869, Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
had implicitly predicted the existence of a heavier analog of titanium and zirconium. At the time of his formulation in 1871, Mendeleev believed that the elements were ordered by their atomic masses and placed lanthanum (element 57) in the spot below zirconium. The exact placement of the elements and the location of missing elements was done by determining the specific weight of the elements and comparing the chemical and physical properties.
The X-ray spectroscopy done by Henry Moseley in 1914 showed a direct dependency between spectral line and effective nuclear charge. This led to the nuclear charge, or atomic number of an element, being used to ascertain its place within the periodic table. With this method, Moseley determined the number of lanthanides and showed the gaps in the atomic number sequence at numbers 43, 61, 72, and 75.
The discovery of the gaps led to an extensive search for the missing elements. In 1914, several people claimed the discovery after Henry Moseley predicted the gap in the periodic table for the then-undiscovered element 72. Georges Urbain asserted that he found element 72 in the rare earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silv ...
s in 1907 and published his results on ''celtium'' in 1911. Neither the spectra nor the chemical behavior he claimed matched with the element found later, and therefore his claim was turned down after a long-standing controversy. The controversy was partly because the chemists favored the chemical techniques which led to the discovery of ''celtium'', while the physicists relied on the use of the new X-ray spectroscopy method that proved that the substances discovered by Urbain did not contain element 72. In 1921, Charles R. BuryKragh, Helge. “Niels Bohr’s Second Atomic Theory.” Historical Studies in the Physical Sciences, vol. 10, University of California Press, 1979, pp. 123–86, https://doi.org/10.2307/27757389. suggested that element 72 should resemble zirconium and therefore was not part of the rare earth elements group. By early 1923, Niels Bohr and others agreed with Bury. These suggestions were based on Bohr's theories of the atom which were identical to chemist Charles Bury, the X-ray spectroscopy of Moseley, and the chemical arguments of Friedrich Paneth
Friedrich Adolf Paneth (31 August 1887 – 17 September 1958) was an Austrian-born British chemist. Fleeing the Nazis, he escaped to Britain. He became a naturalized British citizen in 1939. After the war, Paneth returned to Germany to b ...
.
Encouraged by these suggestions and by the reappearance in 1922 of Urbain's claims that element 72 was a rare earth element discovered in 1911, Dirk Coster and Georg von Hevesy were motivated to search for the new element in zirconium ores. Hafnium was discovered by the two in 1923 in Copenhagen, Denmark, validating the original 1869 prediction of Mendeleev. It was ultimately found in zircon in Norway through X-ray spectroscopy analysis. The place where the discovery took place led to the element being named for the Latin name for "Copenhagen", ''Hafnia'', the home town of Niels Bohr. Today, the Faculty of Science of the University of Copenhagen uses in its seal a stylized image of the hafnium atom.
Hafnium was separated from zirconium through repeated recrystallization of the double ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary a ...
or potassium fluorides by Valdemar Thal Jantzen and von Hevesey. Anton Eduard van Arkel
Anton Eduard van Arkel, (19 November 1893 – 14 March 1976) was a Dutch chemist.
Van Arkel suggested the names "pnictogen" and "pnictide" to refer to chemical elements in group 15 (the nitrogen group or nitrogen family) of the periodic table.
...
and Jan Hendrik de Boer were the first to prepare metallic hafnium by passing hafnium tetraiodide vapor over a heated tungsten filament in 1924. This process for differential purification of zirconium and hafnium is still in use today.
In 1923, six predicted elements were still missing from the periodic table: 43 ( technetium), 61 (promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
), 85 ( astatine), and 87 (francium
Francium is a chemical element with the symbol Fr and atomic number 87. It is extremely radioactive; its most stable isotope, francium-223 (originally called actinium K after the natural decay chain it appears in), has a half-life of only 22&nb ...
) are radioactive elements and are only present in trace amounts in the environment, thus making elements 75 ( rhenium) and 72 (hafnium) the last two unknown non-radioactive elements.
Applications
Most of the hafnium produced is used in the manufacture ofcontrol rod
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron, cadmium, silver, hafnium, or indium, that are capable of absorbing ...
s for nuclear reactors.
Several details contribute to the fact that there are only a few technical uses for hafnium: First, the close similarity between hafnium and zirconium makes it possible to use the more abundant zirconium for most applications; second, hafnium was first available as pure metal after the use in the nuclear industry for hafnium-free zirconium in the late 1950s. Furthermore, the low abundance and difficult separation techniques necessary make it a scarce commodity. When the demand for hafnium-free zirconium dropped following the Fukushima disaster, the price of hafnium increased sharply from around $500–600/kg in 2014 to around $1000/kg in 2015.
Nuclear reactors
The nuclei of several hafnium isotopes can each absorb multiple neutrons. This makes hafnium a good material for use in the control rods for nuclear reactors. Its neutron capture cross section (Capture Resonance Integral Io ≈ 2000 barns) is about 600 times that of zirconium (other elements that are good neutron-absorbers for control rods are cadmium andboron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
). Excellent mechanical properties and exceptional corrosion-resistance properties allow its use in the harsh environment of pressurized water reactors. The German research reactor FRM II
The Research Neutron Source Heinz Maier-Leibnitz (Forschungsreaktor München II or FRM II) (german: Forschungs-Neutronenquelle Heinz Maier-Leibnitz) is a leading German research reactor and neutron source, named in honor of the physicist Heinz ...
uses hafnium as a neutron absorber. It is also common in military reactors, particularly in US naval reactors, but seldom found in civilian ones, the first core of the Shippingport Atomic Power Station (a conversion of a naval reactor) being a notable exception.
Alloys
 Hafnium is used in alloys with iron, titanium,
Hafnium is used in alloys with iron, titanium, niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
, tantalum, and other metals. An alloy used for liquid-rocket thruster nozzles, for example the main engine of the Apollo Lunar Module
The Apollo Lunar Module (LM ), originally designated the Lunar Excursion Module (LEM), was the lunar lander spacecraft that was flown between lunar orbit and the Moon's surface during the United States' Apollo program. It was the first crewed ...
s, is C103 which consists of 89% niobium, 10% hafnium and 1% titanium.
Small additions of hafnium increase the adherence of protective oxide scales on nickel-based alloys. It improves thereby the corrosion resistance especially under cyclic temperature conditions that tend to break oxide scales by inducing thermal stresses between the bulk material and the oxide layer.
Microprocessors
Hafnium-based compounds are employed ingate
A gate or gateway is a point of entry to or from a space enclosed by walls. The word derived from old Norse "gat" meaning road or path; But other terms include ''yett and port''. The concept originally referred to the gap or hole in the wall ...
insulators in the 45 nm generation of integrated circuits from Intel, IBM and others. Hafnium oxide-based compounds are practical high-k dielectrics, allowing reduction of the gate leakage current which improves performance at such scales.
Isotope geochemistry
Isotopes of hafnium and lutetium (along with ytterbium) are also used in isotope geochemistry andgeochronological
Geochronology is the science of determining the age of rocks, fossils, and sediments using signatures inherent in the rocks themselves. Absolute geochronology can be accomplished through radioactive isotopes, whereas relative geochronology is pr ...
applications, in lutetium-hafnium dating. It is often used as a tracer of isotopic evolution of Earth's mantle through time. This is because 176Lu decays to 176Hf with a half-life of approximately 37 billion years.
In most geologic materials, zircon is the dominant host of hafnium (>10,000 ppm) and is often the focus of hafnium studies in geology. Hafnium is readily substituted into the zircon crystal lattice
In geometry and crystallography, a Bravais lattice, named after , is an infinite array of discrete points generated by a set of discrete translation operations described in three dimensional space by
: \mathbf = n_1 \mathbf_1 + n_2 \mathbf_2 + n ...
, and is therefore very resistant to hafnium mobility and contamination. Zircon also has an extremely low Lu/Hf ratio, making any correction for initial lutetium minimal. Although the Lu/Hf system can be used to calculate a " model age", i.e. the time at which it was derived from a given isotopic reservoir such as the depleted mantle, these "ages" do not carry the same geologic significance as do other geochronological techniques as the results often yield isotopic mixtures and thus provide an average age of the material from which it was derived.
Garnet is another mineral that contains appreciable amounts of hafnium to act as a geochronometer. The high and variable Lu/Hf ratios found in garnet make it useful for dating metamorphic events.
Other uses
Due to its heat resistance and its affinity to oxygen and nitrogen, hafnium is a good scavenger for oxygen and nitrogen in gas-filled andincandescent lamp
An incandescent light bulb, incandescent lamp or incandescent light globe is an electric light with a wire filament heated until it glows. The filament is enclosed in a glass bulb with a vacuum or inert gas to protect the filament from oxid ...
s. Hafnium is also used as the electrode in plasma cutting because of its ability to shed electrons into air.
The high energy content of 178m2Hf was the concern of a DARPA-funded program in the US. This program eventually concluded that using the above-mentioned 178m2Hf nuclear isomer of hafnium to construct high-yield weapons with X-ray triggering mechanisms—an application of induced gamma emission—was infeasible because of its expense. See ''hafnium controversy The hafnium controversy is a debate over the possibility of 'triggering' rapid energy releases, via gamma ray emission, from a nuclear isomer of hafnium, 178m2Hf. The energy release is potentially 5 orders of magnitude (100,000 times) more energetic ...
''.
Hafnium metallocene compounds can be prepared from hafnium tetrachloride and various cyclopentadiene
Cyclopentadiene is an organic compound with the chemical formula, formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often ab ...
-type ligand species. Perhaps the simplest hafnium metallocene is hafnocene dichloride. Hafnium metallocenes are part of a large collection of Group 4 transition metal metallocene catalysts that are used worldwide in the production of polyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
resins like polyethylene and polypropylene.
A pyridyl-amidohafnium catalyst can be used for the controlled iso-selective polymerization of propylene which can then be combined with polyethylene to make a much tougher recycled plastic.
Hafnium diselenide
Hafnium is a chemical element with the Symbol (chemistry), symbol Hf and atomic number 72. A lustre (mineralogy), lustrous, silvery gray, tetravalence, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirco ...
is studied in spintronics thanks to its charge density wave and superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
.
Precautions
Care needs to be taken whenmachining
Machining is a process in which a material (often metal) is cut to a desired final shape and size by a controlled material-removal process. The processes that have this common theme are collectively called subtractive manufacturing, which utilizes ...
hafnium because it is pyrophoric—fine particles can spontaneously combust when exposed to air. Compounds that contain this metal are rarely encountered by most people. The pure metal is not considered toxic, but hafnium compounds should be handled as if they were toxic because the ionic forms of metals are normally at greatest risk for toxicity, and limited animal testing has been done for hafnium compounds.
People can be exposed to hafnium in the workplace by breathing it in, swallowing it, skin contact, and eye contact. The Occupational Safety and Health Administration (OSHA) has set the legal limit ( permissible exposure limit) for exposure to hafnium and hafnium compounds in the workplace as TWA 0.5 mg/m3 over an 8-hour workday. The National Institute for Occupational Safety and Health (NIOSH) has set the same recommended exposure limit (REL). At levels of 50 mg/m3, hafnium is immediately dangerous to life and health
The term immediately dangerous to life or health (IDLH) is defined by the US National Institute for Occupational Safety and Health (NIOSH) as exposure to airborne contaminants that is "likely to cause death or immediate or delayed permanent advers ...
.
See also
* * *References
Literature
External links
Hafnium
at Los Alamos National Laboratory'
periodic table of the elements
at '' The Periodic Table of Videos'' (University of Nottingham)
Hafnium Technical & Safety Data
NLM Hazardous Substances Databank – Hafnium, elemental
* Don Clark
Intel Shifts from Silicon to Lift Chip Performance
- WSJ, 2007
* ttps://www.cdc.gov/niosh/npg/npgd0309.html CDC - NIOSH Pocket Guide to Chemical Hazards* https://colnect.com/en/coins/list/composition/168-Hafnium {{Good article Chemical elements Transition metals Neutron poisons 1923 in science Chemical elements with hexagonal close-packed structure