Glycosyl Transferase on:
[Wikipedia]
[Google]
[Amazon]
Glycosyltransferases (GTFs, Gtfs) are enzymes (
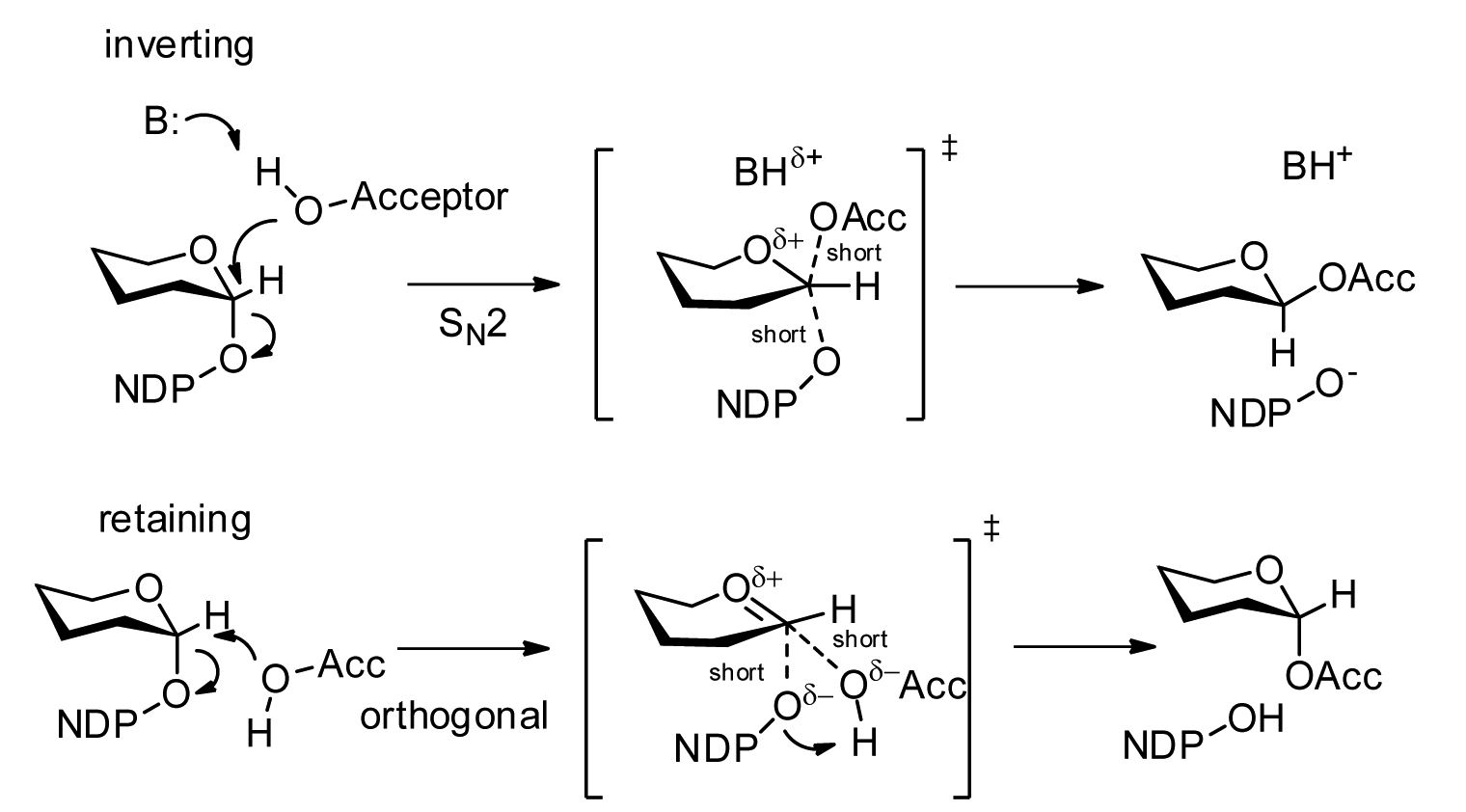 Glycosyltransferases can be segregated into "retaining" or "inverting" enzymes according to whether the stereochemistry of the donor's anomeric bond is retained (α→α) or inverted (α→β) during the transfer. The inverting mechanism is straightforward, requiring a single nucleophilic attack from the accepting atom to invert stereochemistry.
The retaining mechanism has been a matter of debate, but there exists strong evidence against a double displacement mechanism (which would cause two inversions about the anomeric carbon for a net retention of stereochemistry) or a dissociative mechanism (a prevalent variant of which was known as SNi). An "orthogonal associative" mechanism has been proposed which, akin to the inverting enzymes, requires only a single nucleophilic attack from an acceptor from a non-linear angle (as observed in many crystal structures) to achieve anomer retention.
Glycosyltransferases can be segregated into "retaining" or "inverting" enzymes according to whether the stereochemistry of the donor's anomeric bond is retained (α→α) or inverted (α→β) during the transfer. The inverting mechanism is straightforward, requiring a single nucleophilic attack from the accepting atom to invert stereochemistry.
The retaining mechanism has been a matter of debate, but there exists strong evidence against a double displacement mechanism (which would cause two inversions about the anomeric carbon for a net retention of stereochemistry) or a dissociative mechanism (a prevalent variant of which was known as SNi). An "orthogonal associative" mechanism has been proposed which, akin to the inverting enzymes, requires only a single nucleophilic attack from an acceptor from a non-linear angle (as observed in many crystal structures) to achieve anomer retention.
EC 2.4
This list contains a list of EC numbers for the second group, EC 2, transferases, placed in numerical order as determined by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology. All official information is ...
) that establish natural glycosidic linkages. They catalyze the transfer of saccharide moieties from an activated nucleotide sugar (also known as the " glycosyl donor") to a nucleophilic glycosyl acceptor molecule, the nucleophile of which can be oxygen- carbon-, nitrogen-, or sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula ...
-based.
The result of glycosyl transfer can be a carbohydrate, glycoside, oligosaccharide, or a polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wa ...
. Some glycosyltransferases catalyse transfer to inorganic phosphate or water. Glycosyl transfer can also occur to protein residues, usually to tyrosine, serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
, or threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), a carboxyl group (which is in the deprotonated −COO� ...
to give O-linked glycoprotein
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycos ...
s, or to asparagine to give N-linked glycoproteins. Mannosyl groups may be transferred to tryptophan to generate C-mannosyl tryptophan, which is relatively abundant in eukaryotes. Transferases may also use lipids as an acceptor, forming glycolipids, and even use lipid-linked sugar phosphate donors, such as dolichol phosphates in eukaryotic organism, or undecaprenyl phosphate
Undecaprenyl phosphate (UP), also known lipid-P, bactoprenol and C55-P., is a molecule with the primary function of trafficking polysaccharides across the cell membrane, largely contributing to the overall structure of the cell wall in Gram-posit ...
in bacteria.
Glycosyltransferases that use sugar nucleotide donors are Leloir enzymes, after Luis F. Leloir
Luis Federico Leloir (September 6, 1906 – December 2, 1987) was an Argentine physician and biochemist who received the 1970 Nobel Prize in Chemistry for his discovery of the metabolic pathways in lactose. Although born in France, Leloir r ...
, the scientist who discovered the first sugar nucleotide and who received the 1970 Nobel Prize in Chemistry for his work on carbohydrate metabolism. Glycosyltransferases that use non-nucleotide donors such as dolichol or polyprenol pyrophosphate are non-Leloir glycosyltransferases.
Mammals use only 9 sugar nucleotide donors for glycosyltransferases: UDP-glucose, UDP-galactose, UDP-GlcNAc
Uridine diphosphate ''N''-acetylglucosamine or UDP-GlcNAc is a nucleotide sugar and a coenzyme in metabolism. It is used by glycosyltransferases to transfer ''N''-acetylglucosamine residues to substrates. D-Glucosamine is made naturally in the f ...
, UDP-GalNAc, UDP-xylose, UDP-glucuronic acid, GDP-mannose, GDP-fucose, and CMP-sialic acid. The phosphate(s) of these donor molecules are usually coordinated by divalent cations such as manganese, however metal independent enzymes exist.
Many glycosyltransferases are single-pass transmembrane protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the orga ...
s, and they are usually anchored to membranes of Golgi apparatus
Mechanism
 Glycosyltransferases can be segregated into "retaining" or "inverting" enzymes according to whether the stereochemistry of the donor's anomeric bond is retained (α→α) or inverted (α→β) during the transfer. The inverting mechanism is straightforward, requiring a single nucleophilic attack from the accepting atom to invert stereochemistry.
The retaining mechanism has been a matter of debate, but there exists strong evidence against a double displacement mechanism (which would cause two inversions about the anomeric carbon for a net retention of stereochemistry) or a dissociative mechanism (a prevalent variant of which was known as SNi). An "orthogonal associative" mechanism has been proposed which, akin to the inverting enzymes, requires only a single nucleophilic attack from an acceptor from a non-linear angle (as observed in many crystal structures) to achieve anomer retention.
Glycosyltransferases can be segregated into "retaining" or "inverting" enzymes according to whether the stereochemistry of the donor's anomeric bond is retained (α→α) or inverted (α→β) during the transfer. The inverting mechanism is straightforward, requiring a single nucleophilic attack from the accepting atom to invert stereochemistry.
The retaining mechanism has been a matter of debate, but there exists strong evidence against a double displacement mechanism (which would cause two inversions about the anomeric carbon for a net retention of stereochemistry) or a dissociative mechanism (a prevalent variant of which was known as SNi). An "orthogonal associative" mechanism has been proposed which, akin to the inverting enzymes, requires only a single nucleophilic attack from an acceptor from a non-linear angle (as observed in many crystal structures) to achieve anomer retention.
Reaction reversibility
The recent discovery of the reversibility of many reactions catalyzed by inverting glycosyltransferases served as a paradigm shift in the field and raises questions regarding the designation of sugar nucleotides as 'activated' donors.Classification by sequence
Sequence-based classification methods have proven to be a powerful way of generating hypotheses for protein function based on sequence alignment to related proteins. The carbohydrate-active enzyme database presents a sequence-based classification of glycosyltransferases into over 90 families. The same three-dimensional fold is expected to occur within each of the families.Structure
In contrast to the diversity of 3D structures observed for glycoside hydrolases, glycosyltransferase have a much smaller range of structures. In fact, according to theStructural Classification of Proteins
The Structural Classification of Proteins (SCOP) database is a largely manual classification of protein structural domains based on similarities of their structures and amino acid sequences. A motivation for this classification is to determine t ...
database, only three different folds have been observed for glycosyltransferases Very recently, a new glycosyltransferase fold was identified for the glycosyltransferases involved in the biosynthesis of the NAG-NAM polymer backbone of peptidoglycan.
Inhibitors
Many inhibitors of glycosyltransferases are known. Some of these are natural products, such as moenomycin, an inhibitor of peptidoglycan glycosyltransferases, the nikkomycins, inhibitors of chitin synthase, and the echinocandins, inhibitors of fungal β-1,3-glucan synthases. Some glycosyltransferase inhibitors are of use as drugs or antibiotics. Moenomycin is used in animal feed as a growth promoter.Caspofungin
Caspofungin (INN) (brand name Cancidas) is a lipopeptide antifungal drug from Merck & Co., Inc. discovered by James Balkovec, Regina Black and Frances A. Bouffard. It is a member of a new class of antifungals termed the echinocandins. It wor ...
has been developed from the echinocandins and is in use as an antifungal agent. Ethambutol is an inhibitor of mycobacterial arabinotransferases and is used for the treatment of tuberculosis. Lufenuron is an inhibitor of insect chitin syntheses and is used to control fleas in animals. Imidazolium-based synthetic inhibitors of glycosyltransferases have been designed for use as antimicrobial and antiseptic agents.
Determinant of blood type
The ABO blood group system is determined by what type of glycosyltransferases are expressed in the body. The ABO gene locus expressing the glycosyltransferases has three main allelic forms: A, B, and O. The A allele encodes 1-3-N-acetylgalactosaminyltransferase that bonds α- N-acetylgalactosamine to D-galactose end of H antigen, producing the A antigen. The B allele encodes 1-3-galactosyltransferase that joins α-D-galactose bonded to D-galactose end of H antigen, creating the B antigen. In case of O allele the exon 6 contains a deletion that results in a loss of enzymatic activity. The O allele differs slightly from the A allele by deletion of a single nucleotide - Guanine at position 261. The deletion causes a frameshift and results in translation of an almost entirely different protein that lacks enzymatic activity. This results in H antigen remaining unchanged in case of O groups. The combination of glycosyltransferases by both alleles present in each person determines whether there is an AB, A, B or O blood type.Uses
Glycosyltransferases have been widely used in both the targeted synthesis of specific glycoconjugates as well as the synthesis of differentially glycosylated libraries of drugs, biological probes or natural products in the context ofdrug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.
Historically, drugs were discovered by identifying the active ingredient from traditional remedies or by ...
and drug development (a process known as glycorandomization). Suitable enzymes can be isolated from natural sources or produced recombinantly. As an alternative, whole cell-based systems using either endogenous glycosyl donors or cell-based systems containing cloned and expressed systems for synthesis of glycosyl donors have been developed. In cell-free approaches, the large-scale application of glycosyltransferases for glycoconjugate synthesis has required access to large quantities of the glycosyl donors. On the flip-side, nucleotide recycling systems that allow the resynthesis of glycosyl donors from the released nucleotide have been developed. The nucleotide recycling approach has a further benefit of reducing the amount of nucleotide formed as a by-product, thereby reducing the amount of inhibition caused to the glycosyltransferase of interest – a commonly observed feature of the nucleotide byproduct.
See also
* Carbohydrate chemistry * Chemical glycosylation * Glucuronosyltransferase * Glycogen synthase * Glycosyl acceptor * Glycosyl donor *Glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not al ...
* Oligosaccharyltransferase
References
{{Portal bar, Biology, border=no Carbohydrates Carbohydrate chemistry Transferases EC 2.4 EC 2.4.1 EC 2.4.2 Peripheral membrane proteins Glycobiology