Glycerol dehydrogenase on:
[Wikipedia]
[Google]
[Amazon]
Glycerol dehydrogenase (, also known as NAD+-linked glycerol dehydrogenase, glycerol: NAD+ 2-oxidoreductase, GDH, GlDH, GlyDH) is an 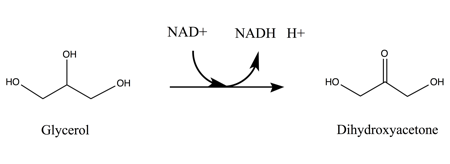 This enzyme is an oxidoreductase, specifically a metal-dependent alcohol dehydrogenase that plays a role in anaerobic glycerol
This enzyme is an oxidoreductase, specifically a metal-dependent alcohol dehydrogenase that plays a role in anaerobic glycerol
 After NAD+ is bound to the enzyme, glycerol substrate binds to the active site in such a way as to have two coordinated interactions between two adjacent hydroxyl groups and the neighboring zinc ion. GlyDH then catalyzes the base-assisted deprotonation of the C2 hydroxyl group, forming an alkoxide. The zinc atom further serves to stabilize the negative charge on the alkoxide intermediate before the excess electron density around the charged oxygen atom shifts to form a double bond with the C2 carbon atom. Hydride is subsequently removed from the secondary carbon and acts as a nucleophile in electron transfer to the NAD+ nicotinamide ring. As a result, the H+ removed by the base is released as a proton into the surrounding solution; followed by the release of the product glycerone, then NADH by GlyDH.
After NAD+ is bound to the enzyme, glycerol substrate binds to the active site in such a way as to have two coordinated interactions between two adjacent hydroxyl groups and the neighboring zinc ion. GlyDH then catalyzes the base-assisted deprotonation of the C2 hydroxyl group, forming an alkoxide. The zinc atom further serves to stabilize the negative charge on the alkoxide intermediate before the excess electron density around the charged oxygen atom shifts to form a double bond with the C2 carbon atom. Hydride is subsequently removed from the secondary carbon and acts as a nucleophile in electron transfer to the NAD+ nicotinamide ring. As a result, the H+ removed by the base is released as a proton into the surrounding solution; followed by the release of the product glycerone, then NADH by GlyDH.
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
in the oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually ut ...
family that utilizes the NAD+ to catalyze
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
the oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
to form glycerone (dihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula .
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, an ...
). This enzyme is an oxidoreductase, specifically a metal-dependent alcohol dehydrogenase that plays a role in anaerobic glycerol
This enzyme is an oxidoreductase, specifically a metal-dependent alcohol dehydrogenase that plays a role in anaerobic glycerol metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cell ...
and has been isolated from a number of bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
, including ''Enterobacter aerogenes,'' ''Klebsiella aerogenes,'' ''Streptococcus faecalis,'' ''Erwinia aeroidea,'' ''Bacillus megaterium,'' and ''Bacillus stearothermophilus.'' However, most studies of glycerol dehydrogenase have been performed in ''Bacillus stearothermophilus
''Geobacillus stearothermophilus'' (previously ''Bacillus stearothermophilus'') is a rod-shaped, Gram-positive bacterium and a member of the phylum Bacillota. The bacterium is a thermophile and is widely distributed in soil, hot springs, ocean s ...
,'' ''(B. stearothermophilus)'' due to its thermostability
In materials science and molecular biology, thermostability is the ability of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature.
...
and the following structural and functional information will, therefore, refer primarily to the characterization of the enzyme in this bacterium.
Structure
Glycerol dehydrogenase is a homooctamer composed of eight identicalmonomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
subunits made up of a single polypeptide chain of 370 amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
(molecular weight 42,000 Da). Each subunit contains 9 beta sheets and 14 alpha helices within two distinct domains (N-terminal, residues 1-162 and C-terminal, residues 163-370). The deep cleft formed between these two domains serves as the enzyme’s active site. This active site consists of one bound metal ion, one NAD+ nicotinamide ring binding site, and a substrate binding site.
Research into the structure of ''B. stearothermophilus'' shows that the active site contains a divalent cation—zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
ion, Zn2+. This zinc ion forms tetrahedral dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
interactions between the amino acid residues Asp173, His256, and His274 as well as a water molecule.
The NAD+ binding site, resembling the Rossmann fold
The Rossmann fold is a tertiary fold found in proteins that bind nucleotides, such as enzyme cofactors FAD, NAD+, and NADP+. This fold is composed of alternating beta strands and alpha helical segments where the beta strands are hydrogen bonded ...
within the N-terminal domain, extends from the surface of the enzyme to the cleft containing the active site. The nicotinamide ring (the active region of NAD+) binds in a pocket of the cleft consisting of the residues Asp100, Asp123, Ala124, Ser127, Leu129, Val131, Asp173, His174, and Phe247.
Finally, the substrate binding site consists of the residues Asp123, His256, His274 as well as a water molecule.
Function
Encoded by the gene gldA, the enzyme glycerol dehydrogenase, GlyDH catalyzes the oxidation of glycerol to glycerone. Unlike more common pathways utilizing glycerol, GlyDH effectively oxidizes glycerol in anaerobic metabolic pathways under ATP-independent conditions (a useful mechanism in the breakdown of glycerol in bacteria). In addition, GlyDH selectively oxidizes the C2 hydroxyl group to form a ketone rather than a terminal hydroxyl group to form an aldehyde.Mechanism
While the precise mechanism of this specific enzyme has not yet been characterized, kinetic studies support that GlyDH catalysis of thechemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
:glycerol + NAD+ glycerone + NADH + H+
is comparable to those of other alcohol dehydrogenases. Therefore, the following mechanism offers a reasonable representation of glycerol oxidation by NAD+.
 After NAD+ is bound to the enzyme, glycerol substrate binds to the active site in such a way as to have two coordinated interactions between two adjacent hydroxyl groups and the neighboring zinc ion. GlyDH then catalyzes the base-assisted deprotonation of the C2 hydroxyl group, forming an alkoxide. The zinc atom further serves to stabilize the negative charge on the alkoxide intermediate before the excess electron density around the charged oxygen atom shifts to form a double bond with the C2 carbon atom. Hydride is subsequently removed from the secondary carbon and acts as a nucleophile in electron transfer to the NAD+ nicotinamide ring. As a result, the H+ removed by the base is released as a proton into the surrounding solution; followed by the release of the product glycerone, then NADH by GlyDH.
After NAD+ is bound to the enzyme, glycerol substrate binds to the active site in such a way as to have two coordinated interactions between two adjacent hydroxyl groups and the neighboring zinc ion. GlyDH then catalyzes the base-assisted deprotonation of the C2 hydroxyl group, forming an alkoxide. The zinc atom further serves to stabilize the negative charge on the alkoxide intermediate before the excess electron density around the charged oxygen atom shifts to form a double bond with the C2 carbon atom. Hydride is subsequently removed from the secondary carbon and acts as a nucleophile in electron transfer to the NAD+ nicotinamide ring. As a result, the H+ removed by the base is released as a proton into the surrounding solution; followed by the release of the product glycerone, then NADH by GlyDH.
Industrial implications
As a result of increasing biodiesel production, formation of the byproduct, crude glycerol, has also increased. While glycerol is commonly used in food, pharmaceuticals, cosmetics, and other industries, increased production of crude glycerol has become very expensive to purify and utilize in these industries. Because of this, researchers are interested in finding new economical ways to utilize low-grade glycerol products. Biotechnology is one such technique: using particular enzymes to break down crude glycerol to form products such as 1,3-propanediol, 1,2-propanediol, succinic acid, dihydroxyacetone (glycerone), hydrogen, polyglycerols, and polyesters. As a catalyst for the conversion of glycerol to glycerone, glycerol dehydrogenase is one such enzyme being investigated for this industrial purpose.See also
* Glycerol dehydrogenase (NADP+) *Glycerol dehydrogenase (acceptor)
In enzymology, a glycerol dehydrogenase (acceptor) () is an enzyme that catalyzes the chemical reaction
:glycerol + acceptor \rightleftharpoons glycerone + reduced acceptor
Thus, the two substrates of this enzyme are glycerol and acceptor, w ...
References
;Notes ;Bibliography * * * {{Portal bar, Biology, border=no EC 1.1.1 NADH-dependent enzymes Enzymes of known structure