
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of
evolutionarily-related proteins that are
cell surface receptor
Cell surface receptors (membrane receptors, transmembrane receptors) are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving (binding to) extracellular molecules. They are specialized integr ...
s that detect
molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
s outside the
cell and activate cellular responses. Coupling with
G protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their ...
s, they are called seven-transmembrane receptors because they pass through the
cell membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the ...
seven times.
[ ] Text was copied from this source, which is available under
Text was copied from this source, which is available under
Attribution 2.5 Generic (CC BY 2.5)
license. Ligands can bind either to extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (Rhodopsin-like family). They are all activated by
agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
s although a spontaneous auto-activation of an empty receptor can also be observed.
[
G protein-coupled receptors are found only in ]eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bact ...
s, including yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are estimated to consti ...
, choanoflagellates,odor
An odor (American English) or odour (Commonwealth English; see spelling differences) is caused by one or more volatilized chemical compounds that are generally found in low concentrations that humans and animals can perceive via their sense ...
s, pheromones, hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required ...
s, and neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neur ...
s, and vary in size from small molecules to peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. ...
s to large protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
s. G protein-coupled receptors are involved in many diseases.
There are two principal signal transduction pathways involving the G protein-coupled receptors:
*the cAMP signal pathway and
*the phosphatidylinositol signal pathway.G protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their ...
by exchanging the GDP bound to the G protein for a GTP. The G protein's α subunit, together with the bound GTP, can then dissociate from the β and γ subunits to further affect intracellular signaling proteins or target functional proteins directly depending on the α subunit type ( Gαs, Gαi/o, Gαq/11, Gα12/13).[
]
History and significance
With the determination of the first structure of the complex between a G-protein coupled receptor (GPCR) and a G-protein trimer (Gαβγ) in 2011 a new chapter of GPCR research was opened for structural investigations of global switches with more than one protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
being investigated. The previous breakthroughs involved determination of the crystal structure of the first GPCR, rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduct ...
, in 2000 and the crystal structure of the first GPCR with a diffusible ligand (β2AR) in 2007. How the seven transmembrane helices of a GPCR are arranged into a bundle was suspected based on the low-resolution model of frog rhodopsin from cryo-electron microscopy studies of the two-dimensional crystals. The crystal structure of rhodopsin, that came up three years later, was not a surprise apart from the presence of an additional cytoplasmic helix H8 and a precise location of a loop covering retinal binding site. However, it provided a scaffold which was hoped to be a universal template for homology modeling and drug design for other GPCRs – a notion that proved to be too optimistic.
Seven years later, the crystallization of β2-adrenergic receptor (β2AR) with a diffusible ligand brought surprising results because it revealed quite a different shape of the receptor extracellular side than that of rhodopsin. This area is important because it is responsible for the ligand binding and is targeted by many drugs. Moreover, the ligand binding site was much more spacious than in the rhodopsin structure and was open to the exterior. In the other receptors crystallized shortly afterwards the binding side was even more easily accessible to the ligand. New structures complemented with biochemical investigations uncovered mechanisms of action of molecular switches which modulate the structure of the receptor leading to activation states for agonists or to complete or partial inactivation states for inverse agonists.[
The 2012 ]Nobel Prize in Chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
was awarded to Brian Kobilka and Robert Lefkowitz for their work that was "crucial for understanding how G protein-coupled receptors function".
Classification
 The exact size of the GPCR superfamily is unknown, but at least 831 different
The exact size of the GPCR superfamily is unknown, but at least 831 different human
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, culture, ...
genes (or ~ 4% of the entire protein-coding
The coding region of a gene, also known as the coding sequence (CDS), is the portion of a gene's DNA or RNA that codes for protein. Studying the length, composition, regulation, splicing, structures, and functions of coding regions compared to no ...
genome
In the fields of molecular biology and genetics, a genome is all the genetic information of an organism. It consists of nucleotide sequences of DNA (or RNA in RNA viruses). The nuclear genome includes protein-coding genes and non-coding ...
) have been predicted to code for them from genome sequence analysis.sequence homology
Sequence homology is the biological homology between DNA, RNA, or protein sequences, defined in terms of shared ancestry in the evolutionary history of life. Two segments of DNA can have shared ancestry because of three phenomena: either a sp ...
between classes.
The largest class by far is class A, which accounts for nearly 85% of the GPCR genes. Of class A GPCRs, over half of these are predicted to encode olfactory receptors, while the remaining receptors are liganded by known endogenous compounds or are classified as orphan receptors. Despite the lack of sequence homology between classes, all GPCRs have a common structure and mechanism of signal transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a series of molecular events, most commonly protein phosphorylation catalyzed by protein kinases, which ultimately results in a cellular ...
. The very large rhodopsin A group has been further subdivided into 19 subgroups ( A1-A19).
According to the classical A-F system, GPCRs can be grouped into 6 classes based on sequence homology and functional similarity:
*Class A (or 1) ( Rhodopsin-like)
*Class B (or 2) ( Secretin receptor family)
* Class C (or 3) ( Metabotropic glutamate/pheromone)
*Class D (or 4) ( Fungal mating pheromone receptors)
*Class E (or 5) ( Cyclic AMP receptors)
*Class F (or 6) ( Frizzled/ Smoothened)
More recently, an alternative classification system called GRAFS (Glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
, Rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduct ...
, ''Adhesion'', Frizzled/ Taste2, Secretin
Secretin is a hormone that regulates water homeostasis throughout the body and influences the environment of the duodenum by regulating secretions in the stomach, pancreas, and liver. It is a peptide hormone produced in the S cells of the duoden ...
) has been proposed for vertebrate GPCRs.[
An early study based on available DNA sequence suggested that the human genome encodes roughly 750 G protein-coupled receptors, about 350 of which detect hormones, growth factors, and other endogenous ligands. Approximately 150 of the GPCRs found in the human genome have unknown functions.
Some web-servers and bioinformatics prediction methods]
Physiological roles
GPCRs are involved in a wide variety of physiological processes. Some examples of their physiological roles include:
# The visual sense: The opsin
Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become Retinylidene proteins, but are usually still called opsins regardless. Most pro ...
s use a photoisomerization reaction to translate electromagnetic radiation
In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, which propagate through space and carry momentum and electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible ...
into cellular signals. Rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduct ...
, for example, uses the conversion of ''11-cis''-retinal to ''all-trans''-retinal for this purpose.
# The gustatory sense (taste): GPCRs in taste cells mediate release of gustducin in response to bitter-, umami- and sweet-tasting substances.
# The sense of smell: Receptors of the olfactory epithelium bind odorants (olfactory receptors) and pheromones (vomeronasal receptors)
# Behavioral and mood regulation: Receptors in the mammalian brain
The brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head ( cephalization), usually near organs for special ...
bind several different neurotransmitter
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, any main body part or target cell, may be another neuron, but could also be a gland or muscle cell.
Neur ...
s, including serotonin, dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 8 ...
, histamine
Histamine is an organic nitrogenous compound involved in local immune responses, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Since histamine was discovered in ...
, GABA, and glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
# Regulation of immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as Tumor immunology, cancer cells and objects such ...
activity and inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving im ...
: chemokine
Chemokines (), or chemotactic cytokines, are a family of small cytokines or Cell signaling, signaling proteins secreted by Cell (biology), cells that induce directional movement of leukocytes, as well as other cell types, including endothelial a ...
receptors bind ligands that mediate intercellular communication between cells of the immune system; receptors such as histamine receptors bind inflammatory mediators and engage target cell types in the inflammatory response. GPCRs are also involved in immune-modulation, e. g. regulating interleukin induction or suppressing TLR-induced immune responses from T cells.
# Autonomic nervous system transmission: Both the sympathetic and parasympathetic
The parasympathetic nervous system (PSNS) is one of the three divisions of the autonomic nervous system, the others being the sympathetic nervous system and the enteric nervous system. The enteric nervous system is sometimes considered part o ...
nervous systems are regulated by GPCR pathways, responsible for control of many automatic functions of the body such as blood pressure, heart rate, and digestive processes
# Cell density sensing: A novel GPCR role in regulating cell density sensing.
# Homeostasis modulation (e.g., water balance).metastasis
Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, ...
of some types of tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
s.
# Used in the endocrine system for peptide and amino-acid derivative hormones that bind to GCPRs on the cell membrane of a target cell. This activates cAMP, which in turn activates several kinases, allowing for a cellular response, such as transcription.
Receptor structure
GPCRs are integral membrane protein
An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All ''transmembrane proteins'' are IMPs, but not all IMPs are transmembrane proteins. IMPs comprise a sign ...
s that possess seven membrane-spanning domains or transmembrane helices.cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, s ...
residues that form disulfide bond
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups ...
s to stabilize the receptor structure. Some seven-transmembrane helix proteins ( channelrhodopsin) that resemble GPCRs may contain ion channels, within their protein.
In 2000, the first crystal structure of a mammalian GPCR, that of bovine rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduct ...
(), was solved.transmembrane domain
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs generally adopt an alpha helix topological conformation, although some TMDs such as those in porins can adopt a different conformation. Because the interior of the lipid b ...
s, such as microbial rhodopsin
Microbial rhodopsins, also known as bacterial rhodopsins are retinal-binding proteins that provide light-dependent ion transport and sensory functions in halophilic
and other bacteria. They are integral membrane proteins with seven transmembr ...
s and adiponectin receptors 1 and 2 ( ADIPOR1 and ADIPOR2). However, these 7TMH (7-transmembrane helices) receptors and channels do not associate with G protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their ...
s. In addition, ADIPOR1 and ADIPOR2 are oriented oppositely to GPCRs in the membrane (i.e. GPCRs usually have an extracellular N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
, cytoplasmic C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein i ...
, whereas ADIPORs are inverted).
Structure–function relationships
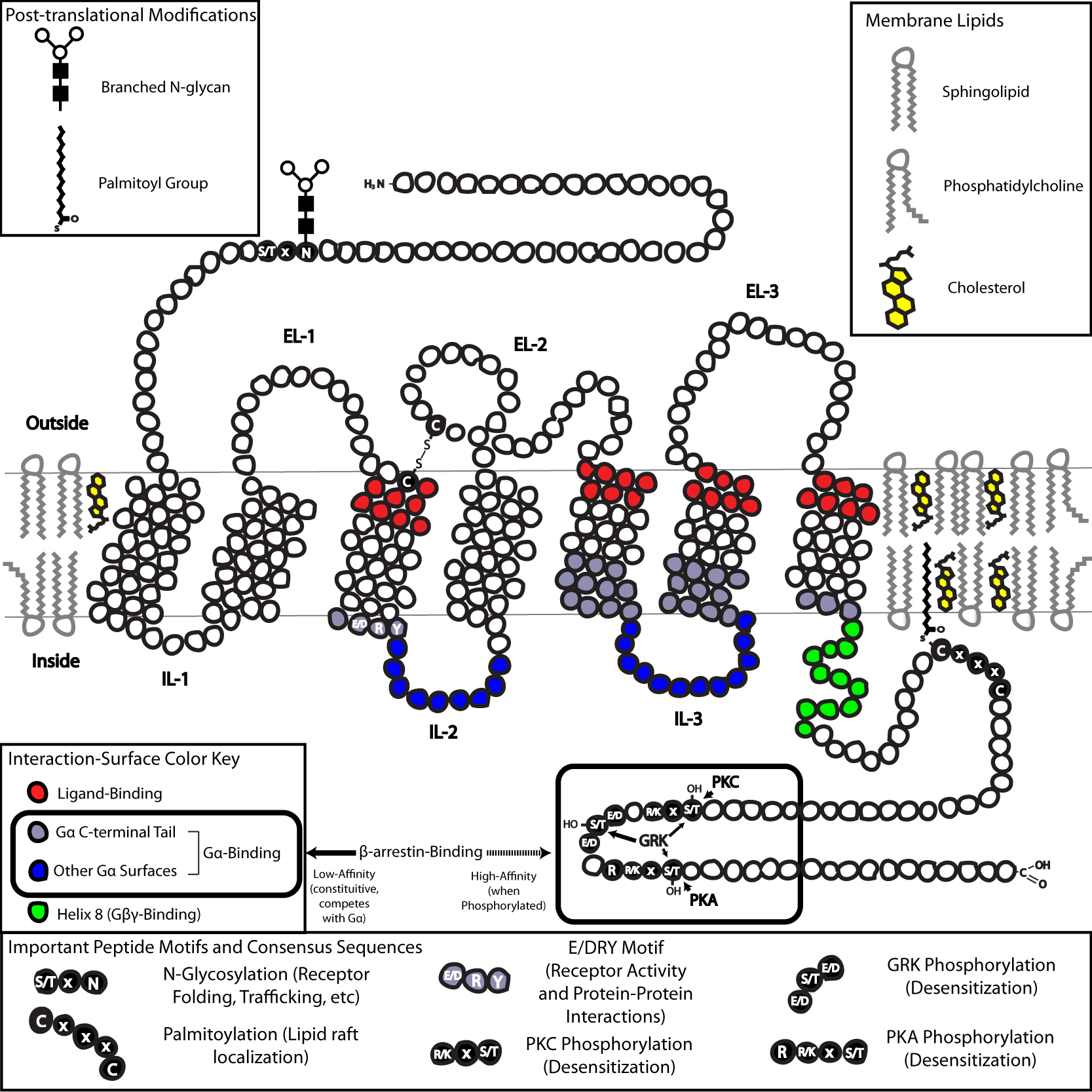 In terms of structure, GPCRs are characterized by an extracellular
In terms of structure, GPCRs are characterized by an extracellular N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the ami ...
, followed by seven transmembrane (7-TM) α-helices (TM-1 to TM-7) connected by three intracellular (IL-1 to IL-3) and three extracellular loops (EL-1 to EL-3), and finally an intracellular C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein i ...
. The GPCR arranges itself into a tertiary structure
Protein tertiary structure is the three dimensional shape of a protein. The tertiary structure will have a single polypeptide chain "backbone" with one or more protein secondary structures, the protein domains. Amino acid side chains may int ...
resembling a barrel, with the seven transmembrane helices forming a cavity within the plasma membrane that serves a ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
-binding domain that is often covered by EL-2. Ligands may also bind elsewhere, however, as is the case for bulkier ligands (e.g., protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
s or large peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. ...
s), which instead interact with the extracellular loops, or, as illustrated by the class C metabotropic glutamate receptors (mGluRs), the N-terminal tail. The class C GPCRs are distinguished by their large N-terminal tail, which also contains a ligand-binding domain. Upon glutamate-binding to an mGluR, the N-terminal tail undergoes a conformational change that leads to its interaction with the residues of the extracellular loops and TM domains. The eventual effect of all three types of agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
-induced activation is a change in the relative orientations of the TM helices (likened to a twisting motion) leading to a wider intracellular surface and "revelation" of residues of the intracellular helices and TM domains crucial to signal transduction function (i.e., G-protein coupling). Inverse agonists and antagonists may also bind to a number of different sites, but the eventual effect must be prevention of this TM helix reorientation.[
The structure of the N- and C-terminal tails of GPCRs may also serve important functions beyond ligand-binding. For example, The C-terminus of M3 muscarinic receptors is sufficient, and the six-amino-acid polybasic (KKKRRK) domain in the C-terminus is necessary for its preassembly with Gq proteins.]sterically
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
prevent G-protein coupling and may recruit other proteins, leading to the creation of signaling complexes involved in extracellular-signal regulated kinase ( ERK) pathway activation or receptor endocytosis
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. E ...
(internalization). As the phosphorylation of these Ser and Thr residues often occurs as a result of GPCR activation, the β-arr-mediated G-protein-decoupling and internalization of GPCRs are important mechanisms of desensitization.cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, s ...
(Cys) residues via addition of hydrophobic acyl groups, and has the effect of targeting the receptor to cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell membr ...
- and sphingolipid-rich microdomains of the plasma membrane called lipid rafts. As many of the downstream transducer and effector molecules of GPCRs (including those involved in negative feedback pathways) are also targeted to lipid rafts, this has the effect of facilitating rapid receptor signaling.
GPCRs respond to extracellular signals mediated by a huge diversity of agonists, ranging from proteins to biogenic amines to protons, but all transduce this signal via a mechanism of G-protein coupling. This is made possible by a guanine-nucleotide exchange factor (guanine nucleotide exchange factor, GEF) domain primarily formed by a combination of IL-2 and IL-3 along with adjacent residues of the associated TM helices.
Mechanism
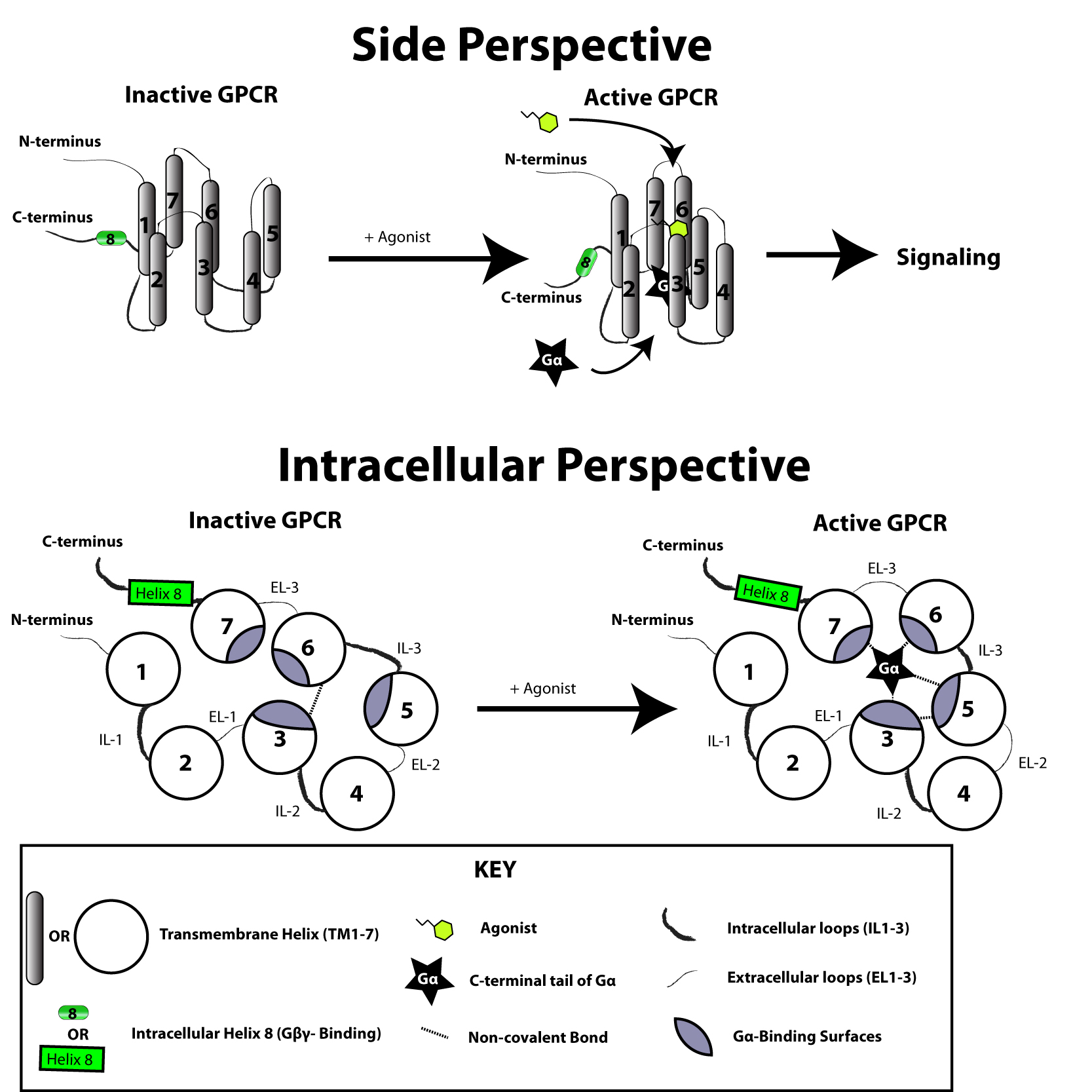 The G protein-coupled receptor is activated by an external signal in the form of a ligand or other signal mediator. This creates a conformational change in the receptor, causing activation of a
The G protein-coupled receptor is activated by an external signal in the form of a ligand or other signal mediator. This creates a conformational change in the receptor, causing activation of a G protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their ...
. Further effect depends on the type of G protein. G proteins are subsequently inactivated by GTPase activating proteins, known as regulator of G protein signaling, RGS proteins.
Ligand binding
GPCRs include one or more receptors for the following ligands:
sensory signal mediators (e.g., light and olfactory stimulatory molecules);
adenosine, bombesin, bradykinin, endothelin, γ-aminobutyric acid (gamma-aminobutyric acid, GABA), hepatocyte growth factor (hepatocyte growth factor, HGF), melanocortins, neuropeptide Y, opioid peptides, opsin
Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become Retinylidene proteins, but are usually still called opsins regardless. Most pro ...
s, somatostatin, growth hormone, GH, tachykinins, members of the vasoactive intestinal peptide family, and vasopressin;
biogenic amines (e.g., dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 8 ...
, epinephrine, norepinephrine, histamine
Histamine is an organic nitrogenous compound involved in local immune responses, as well as regulating physiological functions in the gut and acting as a neurotransmitter for the brain, spinal cord, and uterus. Since histamine was discovered in ...
, serotonin, and melatonin);
glutamate
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can syn ...
(metabotropic effect);
glucagon;
acetylcholine (muscarinic effect);
chemokines;
lipid mediators of inflammation
Inflammation (from la, wikt:en:inflammatio#Latin, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or Irritation, irritants, and is a protective response involving im ...
(e.g., prostaglandins, prostanoids, platelet-activating factor, and leukotrienes);
peptide hormones (e.g., calcitonin, C5a anaphylatoxin, follicle-stimulating hormone [FSH], gonadotropin-releasing hormone [GnRH], neurokinin, thyrotropin-releasing hormone [TRH], and oxytocin);
and endocannabinoids.
GPCRs that act as receptors for stimuli that have not yet been identified are known as orphan receptors.
However, in contrast to other types of receptors that have been studied, wherein ligands bind externally to the membrane, the ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s of GPCRs typically bind within the transmembrane domain. However, protease-activated receptors are activated by cleavage of part of their extracellular domain.
Conformational change
 The signal transduction, transduction of the signal through the membrane by the receptor is not completely understood. It is known that in the inactive state, the GPCR is bound to a heterotrimeric G protein complex. Binding of an agonist to the GPCR results in a conformational change in the receptor that is transmitted to the bound Gα subunit of the heterotrimeric G protein via protein dynamics#Global flexibility: multiple domains, protein domain dynamics. The activated Gα subunit exchanges GTP in place of GDP which in turn triggers the dissociation of Gα subunit from the Gβγ dimer and from the receptor. The dissociated Gα and Gβγ subunits interact with other intracellular proteins to continue the signal transduction cascade while the freed GPCR is able to rebind to another heterotrimeric G protein to form a new complex that is ready to initiate another round of signal transduction.
The signal transduction, transduction of the signal through the membrane by the receptor is not completely understood. It is known that in the inactive state, the GPCR is bound to a heterotrimeric G protein complex. Binding of an agonist to the GPCR results in a conformational change in the receptor that is transmitted to the bound Gα subunit of the heterotrimeric G protein via protein dynamics#Global flexibility: multiple domains, protein domain dynamics. The activated Gα subunit exchanges GTP in place of GDP which in turn triggers the dissociation of Gα subunit from the Gβγ dimer and from the receptor. The dissociated Gα and Gβγ subunits interact with other intracellular proteins to continue the signal transduction cascade while the freed GPCR is able to rebind to another heterotrimeric G protein to form a new complex that is ready to initiate another round of signal transduction.
G-protein activation/deactivation cycle
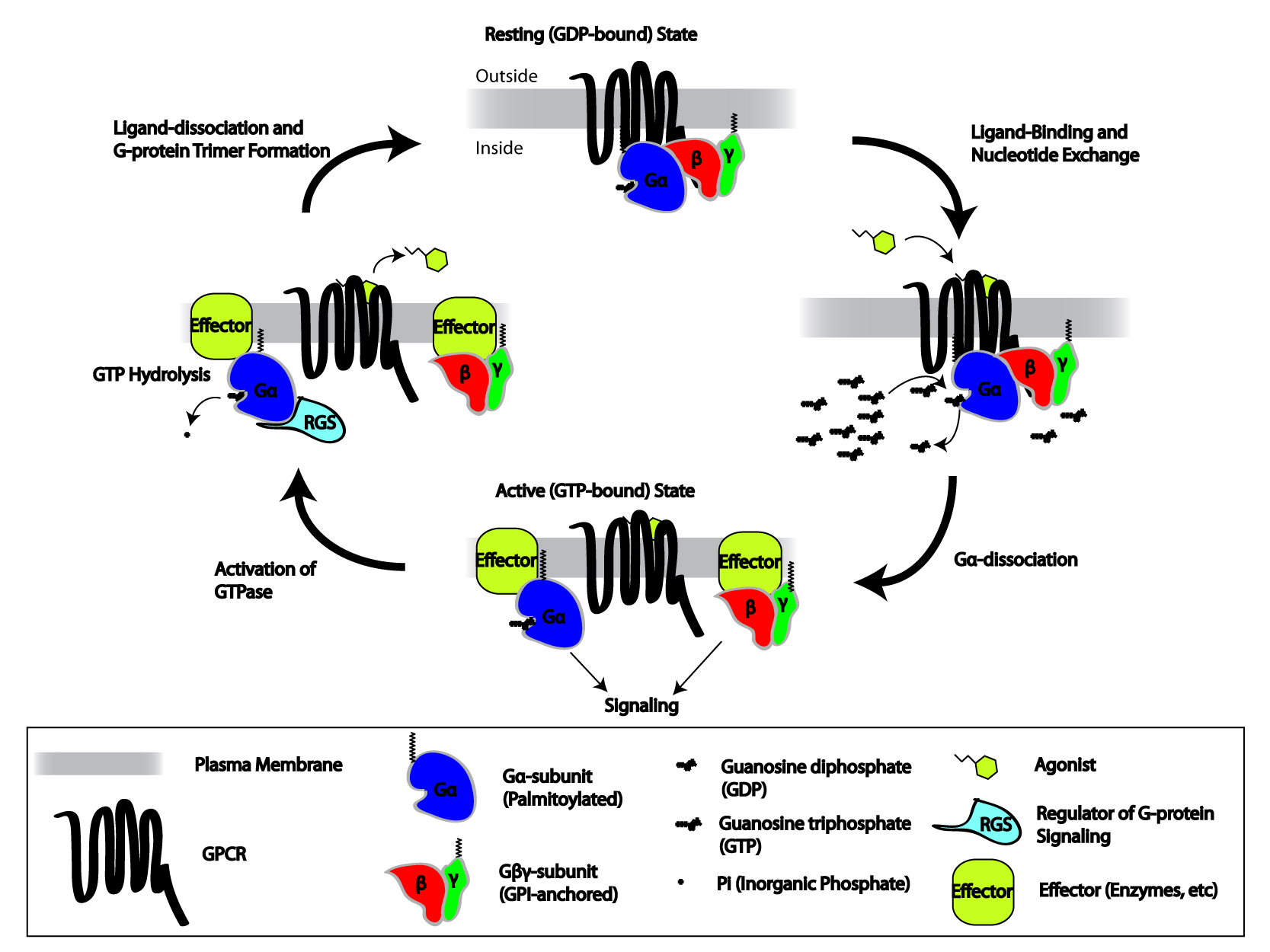 When the receptor is inactive, the guanine nucleotide exchange factor, GEF domain may be bound to an also inactive α-subunit of a heterotrimeric G-protein. These "G-proteins" are a protein trimer, trimer of α, β, and γ subunits (known as Gα, Gβ, and Gγ, respectively) that is rendered inactive when reversibly bound to Guanosine diphosphate (GDP) (or, alternatively, no guanine nucleotide) but active when bound to guanosine triphosphate (GTP). Upon receptor activation, the GEF domain, in turn, allosterically activates the G-protein by facilitating the exchange of a molecule of GDP for GTP at the G-protein's α-subunit. The cell maintains a 10:1 ratio of cytosolic GTP:GDP so exchange for GTP is ensured. At this point, the subunits of the G-protein dissociate from the receptor, as well as each other, to yield a Gα-GTP monomer and a tightly interacting G beta-gamma complex, Gβγ dimer, which are now free to modulate the activity of other intracellular proteins. The extent to which they may diffuse, however, is limited due to the palmitoylation of Gα and the presence of an isoprenoid moiety that has been covalent bond, covalently added to the C-termini of Gγ.
Because Gα also has slow GTP-ase, GTP→GDP hydrolysis capability, the inactive form of the α-subunit (Gα-GDP) is eventually regenerated, thus allowing reassociation with a Gβγ dimer to form the "resting" G-protein, which can again bind to a GPCR and await activation. The rate of GTP hydrolysis is often accelerated due to the actions of another family of allosteric modulating proteins called regulator of G protein signaling, Regulators of G-protein Signaling, or RGS proteins, which are a type of GTPase activating protein, GTPase-Activating Protein, or GAP. In fact, many of the primary Effector (biology), effector proteins (e.g., adenylate cyclases) that become activated/inactivated upon interaction with Gα-GTP also have GAP activity. Thus, even at this early stage in the process, GPCR-initiated signaling has the capacity for self-termination.
When the receptor is inactive, the guanine nucleotide exchange factor, GEF domain may be bound to an also inactive α-subunit of a heterotrimeric G-protein. These "G-proteins" are a protein trimer, trimer of α, β, and γ subunits (known as Gα, Gβ, and Gγ, respectively) that is rendered inactive when reversibly bound to Guanosine diphosphate (GDP) (or, alternatively, no guanine nucleotide) but active when bound to guanosine triphosphate (GTP). Upon receptor activation, the GEF domain, in turn, allosterically activates the G-protein by facilitating the exchange of a molecule of GDP for GTP at the G-protein's α-subunit. The cell maintains a 10:1 ratio of cytosolic GTP:GDP so exchange for GTP is ensured. At this point, the subunits of the G-protein dissociate from the receptor, as well as each other, to yield a Gα-GTP monomer and a tightly interacting G beta-gamma complex, Gβγ dimer, which are now free to modulate the activity of other intracellular proteins. The extent to which they may diffuse, however, is limited due to the palmitoylation of Gα and the presence of an isoprenoid moiety that has been covalent bond, covalently added to the C-termini of Gγ.
Because Gα also has slow GTP-ase, GTP→GDP hydrolysis capability, the inactive form of the α-subunit (Gα-GDP) is eventually regenerated, thus allowing reassociation with a Gβγ dimer to form the "resting" G-protein, which can again bind to a GPCR and await activation. The rate of GTP hydrolysis is often accelerated due to the actions of another family of allosteric modulating proteins called regulator of G protein signaling, Regulators of G-protein Signaling, or RGS proteins, which are a type of GTPase activating protein, GTPase-Activating Protein, or GAP. In fact, many of the primary Effector (biology), effector proteins (e.g., adenylate cyclases) that become activated/inactivated upon interaction with Gα-GTP also have GAP activity. Thus, even at this early stage in the process, GPCR-initiated signaling has the capacity for self-termination.
Crosstalk
 GPCRs downstream signals have been shown to possibly interact with integrin signals, such as PTK2, FAK. Integrin signaling will phosphorylate FAK, which can then decrease GPCR Gαs activity.
GPCRs downstream signals have been shown to possibly interact with integrin signals, such as PTK2, FAK. Integrin signaling will phosphorylate FAK, which can then decrease GPCR Gαs activity.
Signaling
 If a receptor in an active state encounters a
If a receptor in an active state encounters a G protein
G proteins, also known as guanine nucleotide-binding proteins, are a family of proteins that act as molecular switches inside cells, and are involved in transmitting signals from a variety of stimuli outside a cell to its interior. Their ...
, it may activate it. Some evidence suggests that receptors and G proteins are actually pre-coupled.ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
, as well as the availability of transducer molecules. Currently, GPCRs are considered to utilize two primary types of transducers: G-proteins and arrestin, β-arrestins. Because β-arr's have high affinity only to the phosphorylated form of most GPCRs (see above or below), the majority of signaling is ultimately dependent upon G-protein activation. However, the possibility for interaction does allow for G-protein-independent signaling to occur.
G-protein-dependent signaling
There are three main G-protein-mediated signaling pathways, mediated by four class (biology), sub-classes of G-proteins distinguished from each other by sequence homology
Sequence homology is the biological homology between DNA, RNA, or protein sequences, defined in terms of shared ancestry in the evolutionary history of life. Two segments of DNA can have shared ancestry because of three phenomena: either a sp ...
(Gαs, Gαs, Gαi, Gαi/o, Gαq, Gαq/11, and Gα12/13). Each sub-class of G-protein consists of multiple proteins, each the product of multiple genes or splice variant, splice variations that may imbue them with differences ranging from subtle to distinct with regard to signaling properties, but in general they appear reasonably grouped into four classes. Because the signal transducing properties of the various possible G beta-gamma complex, βγ combinations do not appear to radically differ from one another, these classes are defined according to the isoform of their α-subunit.agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the ago ...
may also initiate activation of multiple different G-proteins, as it may be capable of stabilizing more than one conformation of the GPCR's guanine nucleotide exchange factor, GEF domain, even over the course of a single interaction. In addition, a conformation that preferably activates one isoform of Gα may activate another if the preferred is less available. Furthermore, feedback pathways may result in post-translational modification, receptor modifications (e.g., phosphorylation) that alter the G-protein preference. Regardless of these various nuances, the GPCR's preferred coupling partner is usually defined according to the G-protein most obviously activated by the endogenous ligand under most physiological or experimental conditions.
Gα signaling
# The effector of both the Gαs and Gαi/o pathways is the cyclic amp, cyclic-adenosine monophosphate (cAMP)-generating enzyme adenylyl cyclase, adenylate cyclase, or AC. While there are ten different AC gene products in mammals, each with subtle differences in tissue (biology), tissue distribution or function, all catalyze the conversion of cytosolic adenosine triphosphate (ATP) to cAMP, and all are directly stimulated by G-proteins of the Gαs class. In contrast, however, interaction with Gα subunits of the Gαi/o type inhibits AC from generating cAMP. Thus, a GPCR coupled to Gαs counteracts the actions of a GPCR coupled to Gαi/o, and vice versa. The level of cytosolic cAMP may then determine the activity of various cyclic nucleotide-gated ion channel, ion channels as well as members of the Serine/threonine-specific protein kinase, ser/thr-specific protein kinase A (PKA) family. Thus cAMP is considered a second messenger system, second messenger and PKA a secondary effector (biology), effector.
# The effector of the Gαq/11 pathway is phospholipase C, phospholipase C-β (PLCβ), which catalyzes the cleavage of membrane-bound phosphatidylinositol 4,5-bisphosphate (PIP2) into the second messengers inositol trisphosphate, inositol (1,4,5) trisphosphate (IP3) and diglyceride, diacylglycerol (DAG). IP3 acts on inositol trisphosphate receptor, IP3 receptors found in the membrane of the endoplasmic reticulum (ER) to elicit Ca2+, Ca2+ release from the ER, while DAG diffuses along the plasma membrane where it may activate any membrane localized forms of a second ser/thr kinase called protein kinase C (PKC). Since many isoforms of PKC are also activated by increases in intracellular Ca2+, both these pathways can also converge on each other to signal through the same secondary effector. Elevated intracellular Ca2+ also binds and allosterically activates proteins called calmodulins, which in turn tosolic small GTPase, Rho family of GTPases, Rho. Once bound to GTP, Rho can then go on to activate various proteins responsible for cytoskeleton regulation such as Rho-associated protein kinase, Rho-kinase (ROCK). Most GPCRs that couple to Gα12/13 also couple to other sub-classes, often Gαq/11.
Gβγ signaling
The above descriptions ignore the effects of G beta-gamma complex, Gβγ–signalling, which can also be important, in particular in the case of activated Gαi/o-coupled GPCRs. The primary effectors of Gβγ are various ion channels, such as G protein-coupled inwardly-rectifying potassium channel, G-protein-regulated inwardly rectifying K+ channels (GIRKs), P-type calcium channel, P/Q-type calcium channel, Q- and N-type calcium channel, N-type voltage-dependent calcium channel, voltage-gated Ca2+ channels, as well as some isoforms of AC and PLC, along with some PI3K, phosphoinositide-3-kinase (PI3K) isoforms.
G-protein-independent signaling
Although they are classically thought of working only together, GPCRs may signal through G-protein-independent mechanisms, and heterotrimeric G-proteins may play functional roles independent of GPCRs. GPCRs may signal independently through many proteins already mentioned for their roles in G-protein-dependent signaling such as arrestin, β-arrs, G protein-coupled receptor kinase, GRKs, and Src (gene), Srcs. Such signaling has been shown to be physiologically relevant, for example, arrestin, β-arrestin signaling mediated by the chemokine receptor CXCR3 was necessary for full efficacy chemotaxis of activated T cells. In addition, further scaffolding proteins involved in subcellular localization of GPCRs (e.g., PDZ (biology), PDZ-domain-containing proteins) may also act as signal transducers. Most often the effector is a member of the MAPK family.
Examples
In the late 1990s, evidence began accumulating to suggest that some GPCRs are able to signal without G proteins. The MAPK1, ERK2 mitogen-activated protein kinase, a key signal transduction mediator downstream of receptor activation in many pathways, has been shown to be activated in response to cAMP-mediated receptor activation in the slime mold Dictyostelium discoideum, ''D. discoideum'' despite the absence of the associated G protein α- and β-subunits.
In mammalian cells, the much-studied β2-adrenoceptor has been demonstrated to activate the ERK2 pathway after arrestin-mediated uncoupling of G-protein-mediated signaling. Therefore, it seems likely that some mechanisms previously believed related purely to receptor desensitisation are actually examples of receptors switching their signaling pathway, rather than simply being switched off.
In kidney cells, the bradykinin receptor B2 has been shown to interact directly with a protein tyrosine phosphatase. The presence of a tyrosine-phosphorylated immunoreceptor tyrosine-based inhibitory motif, ITIM (immunoreceptor tyrosine-based inhibitory motif) sequence in the B2 receptor is necessary to mediate this interaction and subsequently the antiproliferative effect of bradykinin.
GPCR-independent signaling by heterotrimeric G-proteins
Although it is a relatively immature area of research, it appears that heterotrimeric G-proteins may also take part in non-GPCR signaling. There is evidence for roles as signal transducers in nearly all other types of receptor-mediated signaling, including integrins, receptor tyrosine kinases (RTKs), cytokine receptors (JAK-STAT signaling pathway, JAK/STATs), as well as modulation of various other "accessory" proteins such as guanine nucleotide exchange factor, GEFs, guanosine nucleotide dissociation inhibitors, guanine-nucleotide dissociation inhibitors (GDIs) and protein phosphatases. There may even be specific proteins of these classes whose primary function is as part of GPCR-independent pathways, termed activators of G-protein signalling (AGS). Both the ubiquity of these interactions and the importance of Gα vs. Gβγ subunits to these processes are still unclear.
Details of cAMP and PIP2 pathways


 There are two principal signal transduction pathways involving the G protein-coupled receptors, G protein-linked receptors: the cAMP signal pathway and the phosphatidylinositol signal pathway.
There are two principal signal transduction pathways involving the G protein-coupled receptors, G protein-linked receptors: the cAMP signal pathway and the phosphatidylinositol signal pathway.
cAMP signal pathway
The cAMP signal transduction contains 5 main characters: stimulative hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. Hormones are required ...
receptor (Rs) or inhibitory hormone receptor (Ri); stimulative regulative G-protein (Gs) or inhibitory regulative G-protein (Gi); adenylyl cyclase; protein kinase A (PKA); and cAMP phosphodiesterase.
Stimulative hormone receptor (Rs) is a receptor that can bind with stimulative signal molecules, while inhibitory hormone receptor (Ri) is a receptor that can bind with inhibitory signal molecules.
Stimulative regulative G-protein is a G-protein linked to stimulative hormone receptor (Rs), and its α subunit upon activation could stimulate the activity of an enzyme or other intracellular metabolism. On the contrary, inhibitory regulative G-protein is linked to an inhibitory hormone receptor, and its α subunit upon activation could inhibit the activity of an enzyme or other intracellular metabolism.
Adenylyl cyclase is a 12-transmembrane glycoprotein that catalyzes the conversion of ATP to cAMP with the help of cofactor Mg2+ or Mn2+. The cAMP produced is a second messenger in cellular metabolism and is an allosteric activator of protein kinase A.
Protein kinase A is an important enzyme in cell metabolism due to its ability to regulate cell metabolism by phosphorylating specific committed enzymes in the metabolic pathway. It can also regulate specific gene expression, cellular secretion, and membrane permeability. The protein enzyme contains two catalytic subunits and two regulatory subunits. When there is no cAMP,the complex is inactive. When cAMP binds to the regulatory subunits, their conformation is altered, causing the dissociation of the regulatory subunits, which activates protein kinase A and allows further biological effects.
These signals then can be terminated by cAMP phosphodiesterase, which is an enzyme that degrades cAMP to 5'-AMP and inactivates protein kinase A.
Phosphatidylinositol signal pathway
In the phosphatidylinositol signal pathway, the extracellular signal molecule binds with the G-protein receptor (Gq) on the cell surface and activates phospholipase C, which is located on the cell membrane, plasma membrane. The lipase hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) into two second messengers: inositol trisphosphate, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds with the IP3 receptor in the membrane of the smooth endoplasmic reticulum and mitochondria to open Ca2+ channels. DAG helps activate protein kinase C (PKC), which phosphorylates many other proteins, changing their catalytic activities, leading to cellular responses.
The effects of Ca2+ are also remarkable: it cooperates with DAG in activating PKC and can activate the Ca2+/calmodulin-dependent protein kinase, CaM kinase pathway, in which calcium-modulated protein calmodulin (CaM) binds Ca2+, undergoes a change in conformation, and activates CaM kinase II, which has unique ability to increase its binding affinity to CaM by autophosphorylation, making CaM unavailable for the activation of other enzymes. The kinase then phosphorylates target enzymes, regulating their activities. The two signal pathways are connected together by Ca2+-CaM, which is also a regulatory subunit of adenylyl cyclase and phosphodiesterase in the cAMP signal pathway.
Receptor regulation
GPCRs become desensitized when exposed to their ligand for a long period of time. There are two recognized forms of desensitization: 1) homologous desensitization, in which the activated GPCR is downregulated; and 2) heterologous desensitization, wherein the activated GPCR causes downregulation of a different GPCR. The key reaction of this downregulation is the phosphorylation of the intracellular (or cytoplasmic) receptor domain by protein kinases.
Phosphorylation by cAMP-dependent protein kinases
Cyclic AMP-dependent protein kinases (protein kinase A) are activated by the signal chain coming from the G protein (that was activated by the receptor) via adenylate cyclase and cyclic AMP (cAMP). In a ''feedback mechanism'', these activated kinases phosphorylate the receptor. The longer the receptor remains active the more kinases are activated and the more receptors are phosphorylated. In beta-2 adrenergic receptor, β2-adrenoceptors, this phosphorylation results in the switching of the coupling from the Gs class of G-protein to the Gi alpha subunit, Gi class.
Phosphorylation by GRKs
The G protein-coupled receptor kinases (GRKs) are protein kinases that phosphorylate only active GPCRs.rhodopsin
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduct ...
in retina cells to compensate for exposure to bright light. In many cases, arrestin's binding to the receptor is a prerequisite for translocation. For example, beta-arrestin bound to β2-adrenoreceptors acts as an adaptor for binding with clathrin, and with the beta-subunit of AP2 (clathrin adaptor molecules); thus, the arrestin here acts as a scaffold assembling the components needed for clathrin-mediated endocytosis of β2-adrenoreceptors.
Mechanisms of GPCR signal termination
As mentioned above, G-proteins may terminate their own activation due to their intrinsic GTPase, GTP→GDP hydrolysis capability. However, this reaction proceeds at a slow rate constant, rate (≈.02 times/sec) and, thus, it would take around 50 seconds for any single G-protein to deactivate if other factors did not come into play. Indeed, there are around 30 protein isoform, isoforms of regulator of G protein signaling, RGS proteins that, when bound to Gα through their GTPase activating protein, GAP domain, accelerate the hydrolysis rate to ≈30 times/sec. This 1500-fold increase in rate allows for the cell to respond to external signals with high speed, as well as spatial angular resolution, resolution due to limited amount of second messenger that can be generated and limited distance a G-protein can diffuse in 0.03 seconds. For the most part, the RGS proteins are promiscuous in their ability to activate G-proteins, while which RGS is involved in a given signaling pathway seems more determined by the tissue and GPCR involved than anything else. In addition, RGS proteins have the additional function of increasing the rate of GTP-GDP exchange at GPCRs, (i.e., as a sort of co-GEF) further contributing to the time resolution of GPCR signaling.
In addition, the GPCR may be homologous desensitization, desensitized itself. This can occur as:
# a direct result of receptor theory, ligand occupation, wherein the change in protein conformation, conformation allows recruitment of G protein-coupled receptor kinase, GPCR-Regulating Kinases (GRKs), which go on to phosphorylation, phosphorylate various serine/ threonine residues of IL-3 and the C-terminal tail. Upon GRK phosphorylation, the GPCR's affinity for arrestin, β-arrestin (β-arrestin-1/2 in most tissues) is increased, at which point β-arrestin may bind and act to both sterically
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions ...
hinder G-protein coupling as well as initiate the process of receptor-mediated endocytosis, receptor internalization through clathrin-mediated endocytosis. Because only the liganded receptor is desensitized by this mechanism, it is called homologous desensitization
# the affinity for β-arrestin may be increased in a ligand occupation and GRK-independent manner through phosphorylation of different ser/thr sites (but also of IL-3 and the C-terminal tail) by PKC and PKA. These phosphorylations are often sufficient to impair G-protein coupling on their own as well.
GPCR cellular regulation
Receptor desensitization is mediated through a combination phosphorylation, β-arr binding, and endocytosis as described above. Downregulation occurs when endocytosed receptor is embedded in an endosome that is trafficked to merge with an organelle called a lysosome. Because lysosomal membranes are rich in proton pumps, their interiors have low pH (≈4.8 vs. the pH≈7.2 cytosol), which acts to denature the GPCRs. In addition, lysosomes contain many degradative enzymes, including proteases, which can function only at such low pH, and so the peptide bonds joining the residues of the GPCR together may be cleaved. Whether or not a given receptor is trafficked to a lysosome, detained in endosomes, or trafficked back to the plasma membrane depends on a variety of factors, including receptor type and magnitude of the signal.
GPCR regulation is additionally mediated by gene transcription factors. These factors can increase or decrease gene transcription and thus increase or decrease the generation of new receptors (up- or down-regulation) that travel to the cell membrane.
Receptor oligomerization
G-protein-coupled receptor oligomerisation is a widespread phenomenon. One of the best-studied examples is the metabotropic GABAB receptor, GABAB receptor. This so-called constitutive receptor is formed by heterodimerization of GABBR1, GABABR1 and GABBR2, GABABR2 subunits. Expression of the GABABR1 without the GABABR2 in heterologous systems leads to retention of the subunit in the endoplasmic reticulum. Expression of the GABABR2 subunit alone, meanwhile, leads to surface expression of the subunit, although with no functional activity (i.e., the receptor does not bind agonist and cannot initiate a response following exposure to agonist). Expression of the two subunits together leads to plasma membrane expression of functional receptor. It has been shown that GABABR2 binding to GABABR1 causes masking of a retention signal
Origin and diversification of the superfamily
Signal transduction mediated by the superfamily of GPCRs dates back to the origin of Multicellular organism, multicellularity. Mammalian-like GPCRs are found in fungi, and have been classified according to the GRAFS classification system based on GPCR fingerprints.[ Insect GPCRs appear to be in their own group and Taste2 is identified as descending from ''Rhodopsin''.][ Note that the ''Secretin''/''Adhesion'' split is based on presumed function rather than signature, as the classical Class B (7tm_2, ) is used to identify both in the studies.
]
See also
*G protein-coupled receptors database
*List of MeSH codes (D12.776)
*Metabotropic receptor
*Orphan receptor
*Pepducins, a class of drug candidates targeted at GPCRs
*Receptor activated solely by a synthetic ligand, a technique for control of cell signaling through synthetic GPCRs
*TOG superfamily
References
Further reading
*
*
*
External links
*
GPCR Cell Line
*
* ;
*
*
GPCR-HGmod
, a database of 3D structural models of all human G-protein coupled receptors, built by the GPCR-I-TASSER pipeline
{{G protein-coupled receptors
G protein-coupled receptors,
Biochemistry
Integral membrane proteins
Molecular biology
Protein families
Signal transduction
 G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are  In terms of structure, GPCRs are characterized by an extracellular
In terms of structure, GPCRs are characterized by an extracellular  The G protein-coupled receptor is activated by an external signal in the form of a ligand or other signal mediator. This creates a conformational change in the receptor, causing activation of a
The G protein-coupled receptor is activated by an external signal in the form of a ligand or other signal mediator. This creates a conformational change in the receptor, causing activation of a  The signal transduction, transduction of the signal through the membrane by the receptor is not completely understood. It is known that in the inactive state, the GPCR is bound to a heterotrimeric G protein complex. Binding of an agonist to the GPCR results in a conformational change in the receptor that is transmitted to the bound Gα subunit of the heterotrimeric G protein via protein dynamics#Global flexibility: multiple domains, protein domain dynamics. The activated Gα subunit exchanges GTP in place of GDP which in turn triggers the dissociation of Gα subunit from the Gβγ dimer and from the receptor. The dissociated Gα and Gβγ subunits interact with other intracellular proteins to continue the signal transduction cascade while the freed GPCR is able to rebind to another heterotrimeric G protein to form a new complex that is ready to initiate another round of signal transduction.
It is believed that a receptor molecule exists in a conformational dynamic equilibrium, equilibrium between active and inactive biophysical states. The binding of ligands to the receptor may shift the equilibrium toward the active receptor states. Three types of ligands exist: Agonists are ligands that shift the equilibrium in favour of active states; inverse agonists are ligands that shift the equilibrium in favour of inactive states; and neutral antagonists are ligands that do not affect the equilibrium. It is not yet known how exactly the active and inactive states differ from each other.
The signal transduction, transduction of the signal through the membrane by the receptor is not completely understood. It is known that in the inactive state, the GPCR is bound to a heterotrimeric G protein complex. Binding of an agonist to the GPCR results in a conformational change in the receptor that is transmitted to the bound Gα subunit of the heterotrimeric G protein via protein dynamics#Global flexibility: multiple domains, protein domain dynamics. The activated Gα subunit exchanges GTP in place of GDP which in turn triggers the dissociation of Gα subunit from the Gβγ dimer and from the receptor. The dissociated Gα and Gβγ subunits interact with other intracellular proteins to continue the signal transduction cascade while the freed GPCR is able to rebind to another heterotrimeric G protein to form a new complex that is ready to initiate another round of signal transduction.
It is believed that a receptor molecule exists in a conformational dynamic equilibrium, equilibrium between active and inactive biophysical states. The binding of ligands to the receptor may shift the equilibrium toward the active receptor states. Three types of ligands exist: Agonists are ligands that shift the equilibrium in favour of active states; inverse agonists are ligands that shift the equilibrium in favour of inactive states; and neutral antagonists are ligands that do not affect the equilibrium. It is not yet known how exactly the active and inactive states differ from each other.
 When the receptor is inactive, the guanine nucleotide exchange factor, GEF domain may be bound to an also inactive α-subunit of a heterotrimeric G-protein. These "G-proteins" are a protein trimer, trimer of α, β, and γ subunits (known as Gα, Gβ, and Gγ, respectively) that is rendered inactive when reversibly bound to Guanosine diphosphate (GDP) (or, alternatively, no guanine nucleotide) but active when bound to guanosine triphosphate (GTP). Upon receptor activation, the GEF domain, in turn, allosterically activates the G-protein by facilitating the exchange of a molecule of GDP for GTP at the G-protein's α-subunit. The cell maintains a 10:1 ratio of cytosolic GTP:GDP so exchange for GTP is ensured. At this point, the subunits of the G-protein dissociate from the receptor, as well as each other, to yield a Gα-GTP monomer and a tightly interacting G beta-gamma complex, Gβγ dimer, which are now free to modulate the activity of other intracellular proteins. The extent to which they may diffuse, however, is limited due to the palmitoylation of Gα and the presence of an isoprenoid moiety that has been covalent bond, covalently added to the C-termini of Gγ.
Because Gα also has slow GTP-ase, GTP→GDP hydrolysis capability, the inactive form of the α-subunit (Gα-GDP) is eventually regenerated, thus allowing reassociation with a Gβγ dimer to form the "resting" G-protein, which can again bind to a GPCR and await activation. The rate of GTP hydrolysis is often accelerated due to the actions of another family of allosteric modulating proteins called regulator of G protein signaling, Regulators of G-protein Signaling, or RGS proteins, which are a type of GTPase activating protein, GTPase-Activating Protein, or GAP. In fact, many of the primary Effector (biology), effector proteins (e.g., adenylate cyclases) that become activated/inactivated upon interaction with Gα-GTP also have GAP activity. Thus, even at this early stage in the process, GPCR-initiated signaling has the capacity for self-termination.
When the receptor is inactive, the guanine nucleotide exchange factor, GEF domain may be bound to an also inactive α-subunit of a heterotrimeric G-protein. These "G-proteins" are a protein trimer, trimer of α, β, and γ subunits (known as Gα, Gβ, and Gγ, respectively) that is rendered inactive when reversibly bound to Guanosine diphosphate (GDP) (or, alternatively, no guanine nucleotide) but active when bound to guanosine triphosphate (GTP). Upon receptor activation, the GEF domain, in turn, allosterically activates the G-protein by facilitating the exchange of a molecule of GDP for GTP at the G-protein's α-subunit. The cell maintains a 10:1 ratio of cytosolic GTP:GDP so exchange for GTP is ensured. At this point, the subunits of the G-protein dissociate from the receptor, as well as each other, to yield a Gα-GTP monomer and a tightly interacting G beta-gamma complex, Gβγ dimer, which are now free to modulate the activity of other intracellular proteins. The extent to which they may diffuse, however, is limited due to the palmitoylation of Gα and the presence of an isoprenoid moiety that has been covalent bond, covalently added to the C-termini of Gγ.
Because Gα also has slow GTP-ase, GTP→GDP hydrolysis capability, the inactive form of the α-subunit (Gα-GDP) is eventually regenerated, thus allowing reassociation with a Gβγ dimer to form the "resting" G-protein, which can again bind to a GPCR and await activation. The rate of GTP hydrolysis is often accelerated due to the actions of another family of allosteric modulating proteins called regulator of G protein signaling, Regulators of G-protein Signaling, or RGS proteins, which are a type of GTPase activating protein, GTPase-Activating Protein, or GAP. In fact, many of the primary Effector (biology), effector proteins (e.g., adenylate cyclases) that become activated/inactivated upon interaction with Gα-GTP also have GAP activity. Thus, even at this early stage in the process, GPCR-initiated signaling has the capacity for self-termination.
 GPCRs downstream signals have been shown to possibly interact with integrin signals, such as PTK2, FAK. Integrin signaling will phosphorylate FAK, which can then decrease GPCR Gαs activity.
GPCRs downstream signals have been shown to possibly interact with integrin signals, such as PTK2, FAK. Integrin signaling will phosphorylate FAK, which can then decrease GPCR Gαs activity.
 If a receptor in an active state encounters a
If a receptor in an active state encounters a 
 There are two principal signal transduction pathways involving the G protein-coupled receptors, G protein-linked receptors: the cAMP signal pathway and the phosphatidylinositol signal pathway.
There are two principal signal transduction pathways involving the G protein-coupled receptors, G protein-linked receptors: the cAMP signal pathway and the phosphatidylinositol signal pathway.