Free radical damage to DNA on:
[Wikipedia]
[Google]
[Amazon]
Free radical damage to DNA can occur as a result of exposure to
 Hydroxyl radicals can attack the deoxyribose DNA backbone and bases, potentially causing a plethora of
Hydroxyl radicals can attack the deoxyribose DNA backbone and bases, potentially causing a plethora of
 Hydrogen abstraction from the 1’-deoxyribose carbon by the hydroxyl radical creates a 1 ‘-deoxyribosyl radical. The radical can then react with molecular oxygen, creating a peroxyl radical which can be reduced and dehydrated to yield a 2’-deoxyribonolactone and free base. A deoxyribonolactone is mutagenic and resistant to repair enzymes. Thus, an abasic site is created.
Hydrogen abstraction from the 1’-deoxyribose carbon by the hydroxyl radical creates a 1 ‘-deoxyribosyl radical. The radical can then react with molecular oxygen, creating a peroxyl radical which can be reduced and dehydrated to yield a 2’-deoxyribonolactone and free base. A deoxyribonolactone is mutagenic and resistant to repair enzymes. Thus, an abasic site is created.
 In the presence of DNA, the 1,4-didehydrobenzene diradical abstracts hydrogens from the deoxyribose sugar backbone, predominantly at the C-1’, C-4’ and C-5’ positions. Hydrogen abstraction causes radical formation at the reacted carbon. The carbon radical reacts with molecular oxygen, which leads to a strand break in the DNA through a variety of mechanisms. 1,4-Didehydrobenzene is able to position itself in such a way that it can abstract proximal hydrogens from both strands of DNA. This produces a double-strand break in the DNA, which can lead to cellular
In the presence of DNA, the 1,4-didehydrobenzene diradical abstracts hydrogens from the deoxyribose sugar backbone, predominantly at the C-1’, C-4’ and C-5’ positions. Hydrogen abstraction causes radical formation at the reacted carbon. The carbon radical reacts with molecular oxygen, which leads to a strand break in the DNA through a variety of mechanisms. 1,4-Didehydrobenzene is able to position itself in such a way that it can abstract proximal hydrogens from both strands of DNA. This produces a double-strand break in the DNA, which can lead to cellular  Enediynes generally undergo the Bergman cyclization at temperatures exceeding 200 °C. However, incorporating the enediyne into a 10-membered cyclic hydrocarbon makes the reaction more thermodynamically favorable by releasing the
Enediynes generally undergo the Bergman cyclization at temperatures exceeding 200 °C. However, incorporating the enediyne into a 10-membered cyclic hydrocarbon makes the reaction more thermodynamically favorable by releasing the
 Calicheamicin and other related compounds share several common characteristics. The extended structures attached to the enediyne allow the compound to specifically bind DNA, in most cases to the minor groove of the double helix. Additionally, part of the molecule is known as the “trigger” which, under specific physiological conditions, activates the enediyne, known as the “warhead” and 1,4-didehydrobenzene is generated.
Three classes of enediynes have since been identified: calicheamicin, dynemicin, and
Calicheamicin and other related compounds share several common characteristics. The extended structures attached to the enediyne allow the compound to specifically bind DNA, in most cases to the minor groove of the double helix. Additionally, part of the molecule is known as the “trigger” which, under specific physiological conditions, activates the enediyne, known as the “warhead” and 1,4-didehydrobenzene is generated.
Three classes of enediynes have since been identified: calicheamicin, dynemicin, and  Calicheamicin and the closely related
Calicheamicin and the closely related  Chromoprotein enediynes are characterized by an unstable
Chromoprotein enediynes are characterized by an unstable 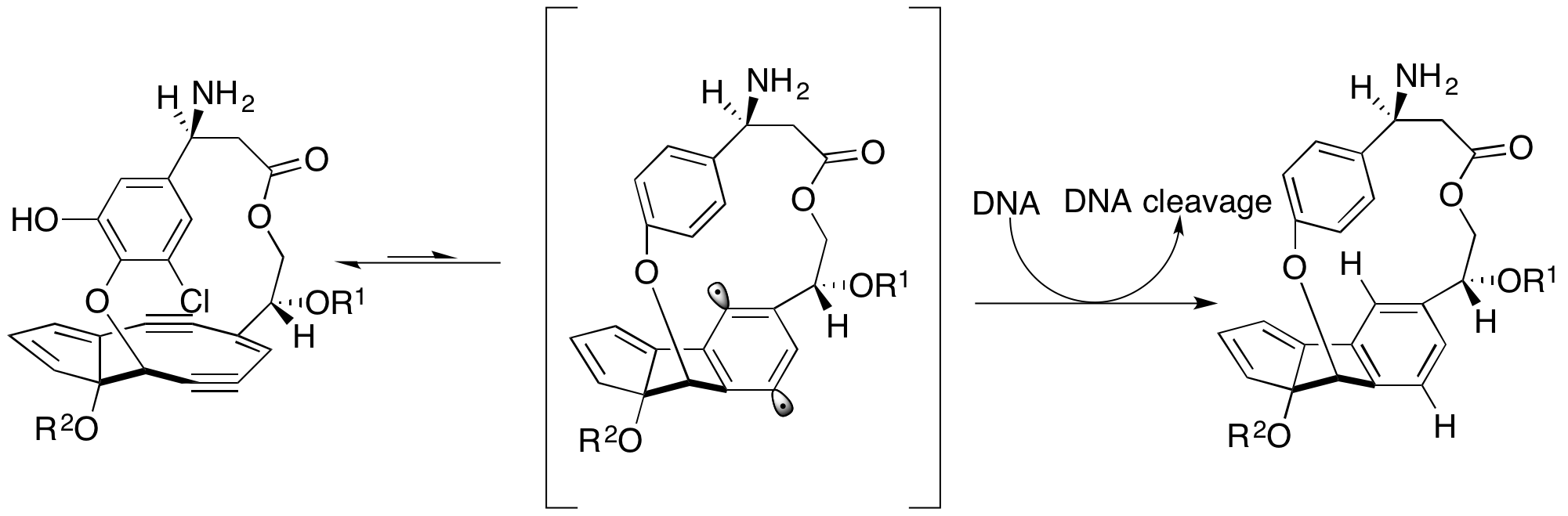 The chromophore is unreactive when bound to the apoprotein. Upon its release, it reacts to form 1,4-didehydrobenzene and subsequently cleaves DNA.
The chromophore is unreactive when bound to the apoprotein. Upon its release, it reacts to form 1,4-didehydrobenzene and subsequently cleaves DNA.
ionizing radiation
Ionizing radiation (or ionising radiation), including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel ...
or to radiomimetic compounds. Damage to DNA as a result of free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
attack is called indirect DNA damage
Indirect, the opposite of direct, may refer to:
*Indirect approach, a battle strategy
*Indirect DNA damage, caused by UV-photons
*Indirect agonist or indirect-acting agonist, a substance that enhances the release or action of an endogenous neurotra ...
because the radicals formed can diffuse throughout the body and affect other organs. Malignant melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the Biological pigment, pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, i ...
can be caused by indirect DNA damage because it is found in parts of the body not exposed to sunlight. DNA is vulnerable to radical attack because of the very labile hydrogens that can be abstracted and the prevalence of double bonds in the DNA bases
Nucleobases, also known as ''nitrogenous bases'' or often simply ''bases'', are nitrogen-containing biological compounds that form nucleosides, which, in turn, are components of nucleotides, with all of these monomers constituting the basic b ...
that free radicals can easily add to.
Damage via radiation exposure
Radiolysis
Radiolysis is the dissociation of molecules by ionizing radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux. The radiation in this context is associated with ionizing radiation; radiolysis is ...
of intracellular water by ionizing radiation creates peroxide
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen p ...
s, which are relatively stable precursors to hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
s. 60%–70% of cellular DNA damage is caused by hydroxyl radicals, yet hydroxyl radicals are so reactive that they can only diffuse one or two molecular diameters before reacting with cellular components. Thus, hydroxyl radicals must be formed immediately adjacent to nucleic acid
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
s in order to react. Radiolysis of water creates peroxides that can act as diffusable, latent forms of hydroxyl radicals. Some metal ions in the vicinity of DNA generate the hydroxyl radicals from peroxide.
::: H2O + ''hν'' → H2O+ + e−
::: H2O + e− → H2O−
::: H2O+ → H+ + OH·
::: H2O− → OH− + H·
::: 2 OH· →H2O2
Free radical damage to DNA is thought to cause mutations that may lead to some cancers.
The Fenton reaction
The Fenton reaction results in the creation of hydroxyl radicals from hydrogen peroxide and an Iron (II) catalyst. Iron(III) is regenerated via theHaber–Weiss reaction The Haber–Weiss reaction generates •OH (hydroxyl radicals) from H2O2 (hydrogen peroxide) and superoxide (•O2−) catalyzed by iron ions. It was first proposed by Fritz Haber and his student Joseph Joshua Weiss in 1932.
This reaction has long ...
. Transition metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
with a free coordination site are capable of reducing peroxides to hydroxyl radicals. Iron is believed to be the metal responsible for the creation of hydroxyl radicals because it exists at the highest concentration of any transition metal in most living organisms. The Fenton reaction is possible because transition metals can exist in more than one oxidation state and their valence electrons may be unpaired, allowing them to participate in one-electron redox reactions.
:::Fe2+ + H2O2 → Fe3+ + OH· + OH−
The creation of hydroxyl radicals by iron(II) catalysis is important because iron(II) can be found coordinated with, and therefore in close proximity to, DNA. This reaction allows for hydrogen peroxide created by radiolysis of water to diffuse to the nucleus and react with Iron (II) to produce hydroxyl radicals, which in turn react with DNA. The location and binding of Iron (II) to DNA may play an important role in determining the substrate and nature of the radical attack on the DNA. The Fenton reaction generates two types of oxidants, Type I and Type II. Type I oxidants are moderately sensitive to peroxides and ethanol. Type I and Type II oxidants preferentially cleave at the specific sequences.
Radical hydroxyl attack
 Hydroxyl radicals can attack the deoxyribose DNA backbone and bases, potentially causing a plethora of
Hydroxyl radicals can attack the deoxyribose DNA backbone and bases, potentially causing a plethora of lesion
A lesion is any damage or abnormal change in the tissue of an organism, usually caused by disease or trauma. ''Lesion'' is derived from the Latin "injury". Lesions may occur in plants as well as animals.
Types
There is no designated classifi ...
s that can be cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'').
Cell physiology
Treating cells ...
or mutagenic
In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in ...
. Cells have developed complex and efficient repair mechanisms to fix the lesions. In the case of free radical attack on DNA, base-excision repair
Base excision repair (BER) is a cellular mechanism, studied in the fields of biochemistry and genetics, that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from t ...
is the repair mechanism used. Hydroxyl radical reactions with the deoxyribose sugar backbone are initiated by hydrogen abstraction from a deoxyribose carbon, and the predominant consequence is eventual strand breakage and base release. The hydroxyl radical reacts with the various hydrogen atoms of the deoxyribose in the order 5′ H > 4′ H > 3′ H ≈ 2′ H ≈ 1′ H. This order of reactivity parallels the exposure to solvent of the deoxyribose hydrogens.
Hydroxyl radicals react with DNA bases via addition to the electron-rich, pi bonds. These pi bonds in the bases are located between C5-C6 of pyrimidine
Pyrimidine (; ) is an aromatic, heterocyclic, organic compound similar to pyridine (). One of the three diazines (six-membered heterocyclics with two nitrogen atoms in the ring), it has nitrogen atoms at positions 1 and 3 in the ring. The other ...
s and N7-C8 in purine
Purine is a heterocyclic compound, heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which includ ...
s. Upon addition of the hydroxyl radical, many stable products can be formed. In general, radical hydroxyl attacks on base moieties do not cause altered sugars or strand breaks except when the modifications labilize the N-glycosyl bond, allowing the formation of baseless sites that are subject to beta-elimination.
Abasic sites
Radical damage through radiomimetic compounds
Radical damage to DNA can also occur through the interaction of DNA with certain natural products known as radiomimetic compounds, molecular compounds which affect DNA in similar ways to radiation exposure. Radiomimetic compounds induce double-strand breaks in DNA via highly specific, concerted free-radical attacks on the deoxyribose moieties in both strands of DNA.General mechanism
Many radiomimetic compounds areenediyne
In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond.
Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne Chemotherapy, anticancer antibiotics). They are ...
s, which undergo the Bergman cyclization
The Masamune-Bergman cyclization or Masamune-Bergman reaction or Masamune-Bergman cycloaromatization is an organic reaction and more specifically a rearrangement reaction taking place when an enediyne is heated in presence of a suitable hydrogen do ...
reaction to produce a 1,4-didehydrobenzene
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes (1,2-didehydroarenes in this case), although 1,3- and 1,4-didehydroarenes are also known. Arynes ar ...
diradical. The 1,4-didehydrobenzene diradical is highly reactive, and will abstract hydrogens from any possible hydrogen-donor.
 In the presence of DNA, the 1,4-didehydrobenzene diradical abstracts hydrogens from the deoxyribose sugar backbone, predominantly at the C-1’, C-4’ and C-5’ positions. Hydrogen abstraction causes radical formation at the reacted carbon. The carbon radical reacts with molecular oxygen, which leads to a strand break in the DNA through a variety of mechanisms. 1,4-Didehydrobenzene is able to position itself in such a way that it can abstract proximal hydrogens from both strands of DNA. This produces a double-strand break in the DNA, which can lead to cellular
In the presence of DNA, the 1,4-didehydrobenzene diradical abstracts hydrogens from the deoxyribose sugar backbone, predominantly at the C-1’, C-4’ and C-5’ positions. Hydrogen abstraction causes radical formation at the reacted carbon. The carbon radical reacts with molecular oxygen, which leads to a strand break in the DNA through a variety of mechanisms. 1,4-Didehydrobenzene is able to position itself in such a way that it can abstract proximal hydrogens from both strands of DNA. This produces a double-strand break in the DNA, which can lead to cellular apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
if not repaired.
 Enediynes generally undergo the Bergman cyclization at temperatures exceeding 200 °C. However, incorporating the enediyne into a 10-membered cyclic hydrocarbon makes the reaction more thermodynamically favorable by releasing the
Enediynes generally undergo the Bergman cyclization at temperatures exceeding 200 °C. However, incorporating the enediyne into a 10-membered cyclic hydrocarbon makes the reaction more thermodynamically favorable by releasing the ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are su ...
of the reactants. This allows for the Bergman cyclization to occur at 37 °C, the biological temperature of humans. Molecules which incorporate enediynes into these larger ring structures have been found to be extremely cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'').
Cell physiology
Treating cells ...
.
Natural products
Enediynes are present in many complicated natural products. They were originally discovered in the early 1980s during a search for new anticancer products produced by microorganisms. Calicheamicin was one of the first such products identified and was originally found in a soil sample taken from Kerrville, Texas. These compounds are synthesized by bacteria as defense mechanisms due to their ability to cleave DNA through the formation of 1,4-didehydrobenzene from the enediyne component of the molecule. Calicheamicin and other related compounds share several common characteristics. The extended structures attached to the enediyne allow the compound to specifically bind DNA, in most cases to the minor groove of the double helix. Additionally, part of the molecule is known as the “trigger” which, under specific physiological conditions, activates the enediyne, known as the “warhead” and 1,4-didehydrobenzene is generated.
Three classes of enediynes have since been identified: calicheamicin, dynemicin, and
Calicheamicin and other related compounds share several common characteristics. The extended structures attached to the enediyne allow the compound to specifically bind DNA, in most cases to the minor groove of the double helix. Additionally, part of the molecule is known as the “trigger” which, under specific physiological conditions, activates the enediyne, known as the “warhead” and 1,4-didehydrobenzene is generated.
Three classes of enediynes have since been identified: calicheamicin, dynemicin, and chromoprotein A chromoprotein is a conjugated protein that contains a pigmented prosthetic group (or cofactor). A common example is haemoglobin, which contains a heme cofactor, which is the iron-containing molecule that makes oxygenated blood appear red. Other e ...
-based products.
The calicheamicin types are defined by a methyl trisulfide group that is involved in triggering the molecule by the following mechanism.
 Calicheamicin and the closely related
Calicheamicin and the closely related esperamicin
The esperamicins are chromoprotein enediyne antitumor antibiotics of bacterial origin. Esperamicin A1 is the most well studied compound in this class. Esperamcin A1 and the related enediyne calicheamicin are the two most potent antitumor agen ...
have been used as anticancer drugs due to their high toxicity and specificity.
Dynemicin and its relatives are characterized by the presence of an anthraquinone
Anthraquinone, also called anthracenedione or dioxoanthracene, is an aromatic organic compound with formula . Isomers include various quinone derivatives. The term anthraquinone however refers to the isomer, 9,10-anthraquinone (IUPAC: 9,10-dioxo ...
and enediyne core. The anthraquinone component allows for specific binding of DNA at the 3’ side of purine bases through intercalation, a site that is different from calicheamicin. Its ability to cleave DNA is greatly increased in the presence of NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NAD ...
and thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
compounds. This compound has also found prominence as an antitumor agent.
chromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
enediyne bound to an apoprotein.
 The chromophore is unreactive when bound to the apoprotein. Upon its release, it reacts to form 1,4-didehydrobenzene and subsequently cleaves DNA.
The chromophore is unreactive when bound to the apoprotein. Upon its release, it reacts to form 1,4-didehydrobenzene and subsequently cleaves DNA.
Antitumor ability
Most enediynes, including the ones listed above, have been used as potent antitumor antibiotics due to their ability to efficiently cleave DNA. Calicheamicin and esperamicin are the two most commonly used types due to their high specificity when binding to DNA, which minimizes unfavorable side reactions. They have been shown to be especially useful for treatingacute myeloid leukemia
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood and interfere with normal blood cell production. Symptoms may includ ...
.
Additionally, calicheamicin is able to cleave DNA at low concentrations, proving to be up to 1000 times more effective than adriamycin
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used toget ...
at combating certain types of tumors. In all cases, cells lack the ability to repair double-stranded DNA breaks, making these compounds especially effective for treating tumor cells.
The free radical mechanism to treat certain types of cancers extends beyond enediynes. Tirapazamine
Tirapazamine (SR-4233) is an experimental anticancer drug that is activated to a toxic radical only at very low levels of oxygen ( hypoxia). Such levels are common in human solid tumors, a phenomenon known as tumor hypoxia. Thus, tirapazamine is ...
generates a free radical under anoxic conditions instead of the trigger mechanism of an enediyne. The free radical then continues on to cleave DNA in a similar manner to 1,4-didehydrobenzene in order to treat cancerous cells. It is currently in Phase III trials.
Evolution of Meiosis
Meiosis
Meiosis (; , since it is a reductional division) is a special type of cell division of germ cells in sexually-reproducing organisms that produces the gametes, such as sperm or egg cells. It involves two rounds of division that ultimately resu ...
is a central feature of sexual reproduction
Sexual reproduction is a type of reproduction that involves a complex life cycle in which a gamete ( haploid reproductive cells, such as a sperm or egg cell) with a single set of chromosomes combines with another gamete to produce a zygote tha ...
in eukaryotes
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
. The need to repair oxidative DNA damage caused by oxidative free radicals has been hypothesized to be a major driving force in the evolution of meiosisHörandl E, Speijer D. How oxygen gave rise to eukaryotic sex. Proc Biol Sci. 2018 Feb 14;285(1872):20172706. doi: 10.1098/rspb.2017.2706. PMID: 29436502; PMCID: PMC5829205
References
{{Reflist DNA DNA repair Molecular genetics