Fluorite Glass on:
[Wikipedia]
[Google]
[Amazon]
Fluorite (also called fluorspar) is the mineral form of calcium fluoride, CaF2. It belongs to the
Mindat.org Currently, the word "fluorspar" is most commonly used for fluorite as the industrial and chemical commodity, while "fluorite" is used mineralogically and in most other senses. In the context of archeology, gemmology, classical studies, and Egyptology, the Latin terms ''murrina'' and ''myrrhina'' refer to fluorite. In book 37 of his '' Naturalis Historia'', Pliny the Elder describes it as a precious stone with purple and white mottling, whose objects carved from it, the Romans prize.
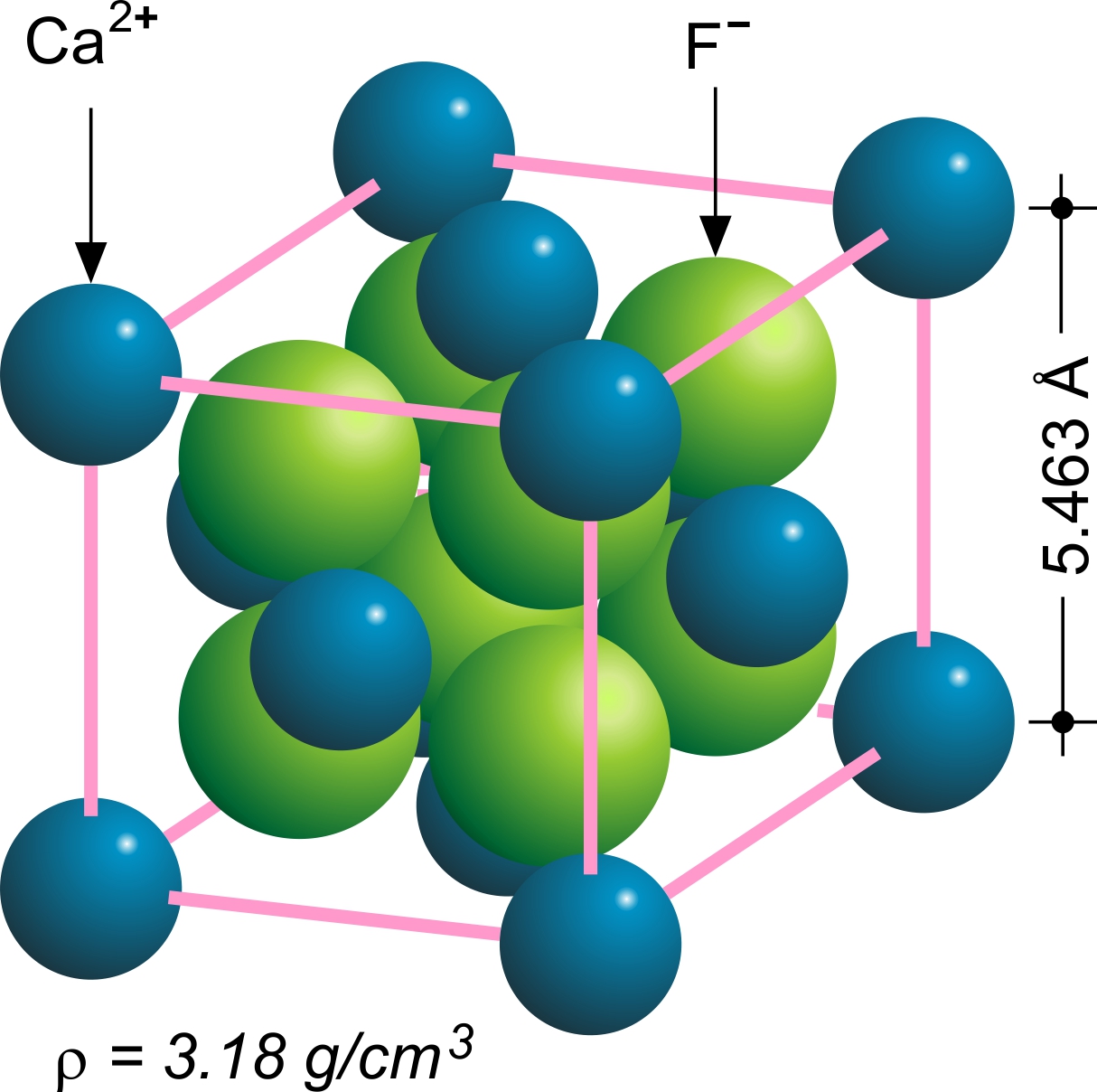 Fluorite crystallizes in a cubic motif. Crystal twinning is common and adds complexity to the observed
Fluorite crystallizes in a cubic motif. Crystal twinning is common and adds complexity to the observed
 Fluorite forms as a late-crystallizing mineral in
Fluorite forms as a late-crystallizing mineral in  In Asturias ( Spain) there are several fluorite deposits known internationally for the quality of the specimens they have yielded. In the area of Berbes, Ribadesella, fluorite appears as cubic crystals, sometimes with dodecahedron modifications, which can reach a size of up to 10 cm of edge, with internal colour zoning, almost always violet in colour. It is associated with quartz and leafy aggregates of baryte. In the ''Emilio'' mine, in Loroñe, Colunga, the fluorite crystals, cubes with small modifications of other figures, are colourless and transparent. They can reach 10 cm of edge. In the ''Moscona'' mine, in Villabona, the fluorite crystals, cubic without modifications of other shapes, are yellow, up to 3 cm of edge. They are associated with large crystals of calcite and barite.
In Asturias ( Spain) there are several fluorite deposits known internationally for the quality of the specimens they have yielded. In the area of Berbes, Ribadesella, fluorite appears as cubic crystals, sometimes with dodecahedron modifications, which can reach a size of up to 10 cm of edge, with internal colour zoning, almost always violet in colour. It is associated with quartz and leafy aggregates of baryte. In the ''Emilio'' mine, in Loroñe, Colunga, the fluorite crystals, cubes with small modifications of other figures, are colourless and transparent. They can reach 10 cm of edge. In the ''Moscona'' mine, in Villabona, the fluorite crystals, cubic without modifications of other shapes, are yellow, up to 3 cm of edge. They are associated with large crystals of calcite and barite.
 George Gabriel Stokes named the phenomenon of ''fluorescence'' from fluorite, in 1852.
Many samples of fluorite exhibit fluorescence under ultraviolet light, a property that takes its name from fluorite. Many minerals, as well as other substances, fluoresce. Fluorescence involves the elevation of electron energy levels by quanta of ultraviolet light, followed by the progressive falling back of the electrons into their previous energy state, releasing quanta of visible light in the process. In fluorite, the visible light emitted is most commonly blue, but red, purple, yellow, green, and white also occur. The fluorescence of fluorite may be due to mineral impurities, such as yttrium and ytterbium, or organic matter, such as volatile hydrocarbons in the crystal lattice. In particular, the blue fluorescence seen in fluorites from certain parts of Great Britain responsible for the naming of the phenomenon of fluorescence itself, has been attributed to the presence of inclusions of divalent
George Gabriel Stokes named the phenomenon of ''fluorescence'' from fluorite, in 1852.
Many samples of fluorite exhibit fluorescence under ultraviolet light, a property that takes its name from fluorite. Many minerals, as well as other substances, fluoresce. Fluorescence involves the elevation of electron energy levels by quanta of ultraviolet light, followed by the progressive falling back of the electrons into their previous energy state, releasing quanta of visible light in the process. In fluorite, the visible light emitted is most commonly blue, but red, purple, yellow, green, and white also occur. The fluorescence of fluorite may be due to mineral impurities, such as yttrium and ytterbium, or organic matter, such as volatile hydrocarbons in the crystal lattice. In particular, the blue fluorescence seen in fluorites from certain parts of Great Britain responsible for the naming of the phenomenon of fluorescence itself, has been attributed to the presence of inclusions of divalent
File:Fluorite-Galena-flu70a.jpg, Pastel green fluorite crystal on galena
File:Fluorite-132158.jpg, A golden yellow with hints of purple fluorite
File:Fluorite-cflo06x.jpg, Freestanding purple fluorite cluster between two quartzes
File:Fluorite-189396.jpg, Light to dark burgundy color fluorite
File:Fluorite-158842.jpg, Transparent teal color fluorite with purple highlights
File:Fluorite-233168.jpg, Grass-green fluorite octahedrons clustered on a quartz-rich matrix
Fluorspar
USGS 2009 Minerals Yearbook

Fluorine finally found in nature , Chemistry World
Rsc.org.
File:Fluorite crystals (Cullen Hall of Gems and Minerals).jpg, Fluorite crystals on display at the Cullen Hall of Gems and Minerals, Houston Museum of Natural Science
File:Fluorite and sphalerite J1.jpg, Fluorite and sphalerite, from Elmwood mine, Smith county, Tennessee, US
File:Fluorite-Quartz-226312.jpg, Translucent ball of botryoidal fluorite perched on a calcite crystal
File:FluoriteBerbes.jpg, Fluorite with baryte, from Berbes Mine, Berbes Mining area, Ribadesella, Asturias, Spain
File:Fluorite - Diana Maria mine, Rogerley quarry, Stanhope, County Durham, England.jpg, Fluorite from Diana Maria mine, Weardale, England, UK
File:FluoriteMaroc.jpg, Fluorite from El Hammam Mine, Meknès Prefecture, Meknès-Tafilalet Region, Morocco
File:Fluorite frog, length 8 cm arp.jpg, Toad carved in fluorite. Length 8 cm (3 in).
Educational article about the different colors of fluorites crystals from Asturias, SpainBarber Cup
an
Crawford Cup
related Roman cups at British Museum {{Authority control Cubic minerals Minerals in space group 225 Evaporite Fluorine minerals Luminescent minerals Industrial minerals Symbols of Illinois
halide mineral
Halide minerals are those minerals with a dominant halide anion (, , and ). Complex halide minerals may also have polyatomic anions.
Examples include the following:
*Atacamite
* Avogadrite (K,Cs)BF
*Bararite (β)
*Bischofite
* Brüggenite ...
s. It crystallizes in isometric
The term ''isometric'' comes from the Greek for "having equal measurement".
isometric may mean:
* Cubic crystal system, also called isometric crystal system
* Isometre, a rhythmic technique in music.
* "Isometric (Intro)", a song by Madeon from ...
cubic habit, although octahedral and more complex isometric forms are not uncommon.
The Mohs scale of mineral hardness, based on scratch hardness comparison, defines value 4 as fluorite.
Pure fluorite is colourless and transparent, both in visible and ultraviolet light, but impurities usually make it a colorful mineral and the stone has ornamental and lapidary uses. Industrially, fluorite is used as a flux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications to physics. For transport ph ...
for smelting, and in the production of certain glasses and enamels. The purest grades of fluorite are a source of fluoride for hydrofluoric acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include th ...
manufacture, which is the intermediate source of most fluorine-containing fine chemicals. Optically clear transparent fluorite lenses have low dispersion, so lenses made from it exhibit less chromatic aberration, making them valuable in microscopes and telescopes. Fluorite optics are also usable in the far-ultraviolet and mid-infrared ranges, where conventional glasses are too opaque for use.
History and etymology
The word ''fluorite'' is derived from the Latin verb ''fluere'', meaning ''to flow''. The mineral is used as aflux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications to physics. For transport ph ...
in iron smelting to decrease the viscosity of slag
Slag is a by-product of smelting (pyrometallurgical) ores and used metals. Broadly, it can be classified as ferrous (by-products of processing iron and steel), ferroalloy (by-product of ferroalloy production) or non-ferrous/base metals (by-prod ...
. The term ''flux'' comes from the Latin adjective ''fluxus'', meaning ''flowing, loose, slack''. The mineral fluorite was originally termed fluorospar and was first discussed in print in a 1530 work ''Bermannvs sive de re metallica dialogus'' ermannus; or a dialogue about the nature of metals by Georgius Agricola, as a mineral noted for its usefulness as a flux. Agricola, a German scientist with expertise in philology, mining, and metallurgy, named fluorspar as a neo-Latinization of the German ''Flussspat'' from ''Fluss'' (stream
A stream is a continuous body of water, body of surface water Current (stream), flowing within the stream bed, bed and bank (geography), banks of a channel (geography), channel. Depending on its location or certain characteristics, a stream ...
, river) and ''Spat'' (meaning a nonmetal
In chemistry, a nonmetal is a chemical element that generally lacks a predominance of metallic properties; they range from colorless gases (like hydrogen) to shiny solids (like carbon, as graphite). The electrons in nonmetals behave differentl ...
lic mineral akin to gypsum, spærstān, '' spear stone'', referring to its crystalline projections).
In 1852, fluorite gave its name to the phenomenon of fluorescence, which is prominent in fluorites from certain locations, due to certain impurities in the crystal. Fluorite also gave the name to its constitutive element fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
.FluoriteMindat.org Currently, the word "fluorspar" is most commonly used for fluorite as the industrial and chemical commodity, while "fluorite" is used mineralogically and in most other senses. In the context of archeology, gemmology, classical studies, and Egyptology, the Latin terms ''murrina'' and ''myrrhina'' refer to fluorite. In book 37 of his '' Naturalis Historia'', Pliny the Elder describes it as a precious stone with purple and white mottling, whose objects carved from it, the Romans prize.
Structure
 Fluorite crystallizes in a cubic motif. Crystal twinning is common and adds complexity to the observed
Fluorite crystallizes in a cubic motif. Crystal twinning is common and adds complexity to the observed crystal habit
In mineralogy, crystal habit is the characteristic external shape of an individual crystal or crystal group. The habit of a crystal is dependent on its crystallographic form and growth conditions, which generally creates irregularities due to l ...
s. Fluorite has four perfect cleavage planes that help produce octahedral fragments. The structural motif adopted by fluorite is so common that the motif is called the fluorite structure. Element substitution for the calcium cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
often includes strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is ex ...
and certain rare-earth elements (REE), such as yttrium and cerium.
Occurrence and mining
 Fluorite forms as a late-crystallizing mineral in
Fluorite forms as a late-crystallizing mineral in felsic
In geology, felsic is a modifier describing igneous rocks that are relatively rich in elements that form feldspar and quartz.Marshak, Stephen, 2009, ''Essentials of Geology,'' W. W. Norton & Company, 3rd ed. It is contrasted with mafic rocks, whi ...
igneous rocks typically through hydrothermal activity. It is particularly common in granitic pegmatites. It may occur as a vein deposit formed through hydrothermal activity particularly in limestones. In such vein deposits it can be associated with galena
Galena, also called lead glance, is the natural mineral form of lead(II) sulfide (PbS). It is the most important ore of lead and an important source of silver.
Galena is one of the most abundant and widely distributed sulfide minerals. It cryst ...
, sphalerite
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in Sedimentary exhalative deposits, sedimen ...
, barite
Baryte, barite or barytes ( or ) is a mineral consisting of barium sulfate ( Ba S O4). Baryte is generally white or colorless, and is the main source of the element barium. The ''baryte group'' consists of baryte, celestine (strontium sulfate), ...
, quartz, and calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on ...
. Fluorite can also be found as a constituent of sedimentary rocks either as grains or as the cementing material in sandstone.
It is a common mineral mainly distributed in South Africa, China, Mexico, Mongolia, the United Kingdom, the United States, Canada, Tanzania, Rwanda and Argentina.
The world reserves of fluorite are estimated at 230 million tonnes (Mt) with the largest deposits being in South Africa (about 41 Mt), Mexico (32 Mt) and China (24 Mt). China is leading the world production with about 3 Mt annually (in 2010), followed by Mexico (1.0 Mt), Mongolia (0.45 Mt), Russia (0.22 Mt), South Africa (0.13 Mt), Spain (0.12 Mt) and Namibia (0.11 Mt).
One of the largest deposits of fluorspar in North America is located on the Burin Peninsula, Newfoundland
Newfoundland and Labrador (; french: Terre-Neuve-et-Labrador; frequently abbreviated as NL) is the easternmost province of Canada, in the country's Atlantic region. The province comprises the island of Newfoundland and the continental region ...
, Canada. The first official recognition of fluorspar in the area was recorded by geologist J.B. Jukes in 1843. He noted an occurrence of "galena" or lead ore and fluoride of lime on the west side of St. Lawrence harbour. It is recorded that interest in the commercial mining of fluorspar began in 1928 with the first ore being extracted in 1933. Eventually, at Iron Springs Mine, the shafts reached depths of . In the St. Lawrence area, the veins are persistent for great lengths and several of them have wide lenses. The area with veins of known workable size comprises about .
In 2018, Canada Fluorspar Inc. commenced mine production again in St. Lawrence; in spring 2019, the company was planned to develop a new shipping port on the west side of Burin Peninsula as a more affordable means of moving their product to markets, and they successfully sent the first shipload of ore from the new port on July 31, 2021. This marks the first time in 30 years that ore has been shipped directly out of St. Lawrence.
Cubic crystals up to 20 cm across have been found at Dalnegorsk
Dalnegorsk (russian: Дальнего́рск, lit. ''far in the mountains'') is a town in Primorsky Krai, Russia. Population:
Name
It was formerly known from its founding in 1897 as Tetyukhe (russian: Те́тюхе; ; literally meaning "riv ...
, Russia. The largest documented single crystal of fluorite was a cube 2.12 meters in size and weighing approximately 16 tonnes.
 In Asturias ( Spain) there are several fluorite deposits known internationally for the quality of the specimens they have yielded. In the area of Berbes, Ribadesella, fluorite appears as cubic crystals, sometimes with dodecahedron modifications, which can reach a size of up to 10 cm of edge, with internal colour zoning, almost always violet in colour. It is associated with quartz and leafy aggregates of baryte. In the ''Emilio'' mine, in Loroñe, Colunga, the fluorite crystals, cubes with small modifications of other figures, are colourless and transparent. They can reach 10 cm of edge. In the ''Moscona'' mine, in Villabona, the fluorite crystals, cubic without modifications of other shapes, are yellow, up to 3 cm of edge. They are associated with large crystals of calcite and barite.
In Asturias ( Spain) there are several fluorite deposits known internationally for the quality of the specimens they have yielded. In the area of Berbes, Ribadesella, fluorite appears as cubic crystals, sometimes with dodecahedron modifications, which can reach a size of up to 10 cm of edge, with internal colour zoning, almost always violet in colour. It is associated with quartz and leafy aggregates of baryte. In the ''Emilio'' mine, in Loroñe, Colunga, the fluorite crystals, cubes with small modifications of other figures, are colourless and transparent. They can reach 10 cm of edge. In the ''Moscona'' mine, in Villabona, the fluorite crystals, cubic without modifications of other shapes, are yellow, up to 3 cm of edge. They are associated with large crystals of calcite and barite.
"Blue John"
One of the most famous of the older-known localities of fluorite is Castleton in Derbyshire, England, where, under the name of "Derbyshire Blue John", purple-blue fluorite was extracted from several mines or caves. During the 19th century, this attractive fluorite was mined for its ornamental value. The mineral Blue John is now scarce, and only a few hundred kilograms are mined each year for ornamental and lapidary use. Mining still takes place inBlue John Cavern
The Blue John Cavern is one of the four show caves in Castleton, Derbyshire, England.
Description
The cavern takes its name from the semi-precious mineral Blue John, which is still mined in small amounts outside the tourist season and mad ...
and Treak Cliff Cavern
Treak Cliff Cavern is a show cave near Castleton in Derbyshire, England. It is part of the Castleton Site of Special Scientific Interest and one of only two sites where the ornamental mineral Blue John is still excavated (the other is the n ...
.
Recently discovered deposits in China have produced fluorite with coloring and banding similar to the classic Blue John stone.
Fluorescence
 George Gabriel Stokes named the phenomenon of ''fluorescence'' from fluorite, in 1852.
Many samples of fluorite exhibit fluorescence under ultraviolet light, a property that takes its name from fluorite. Many minerals, as well as other substances, fluoresce. Fluorescence involves the elevation of electron energy levels by quanta of ultraviolet light, followed by the progressive falling back of the electrons into their previous energy state, releasing quanta of visible light in the process. In fluorite, the visible light emitted is most commonly blue, but red, purple, yellow, green, and white also occur. The fluorescence of fluorite may be due to mineral impurities, such as yttrium and ytterbium, or organic matter, such as volatile hydrocarbons in the crystal lattice. In particular, the blue fluorescence seen in fluorites from certain parts of Great Britain responsible for the naming of the phenomenon of fluorescence itself, has been attributed to the presence of inclusions of divalent
George Gabriel Stokes named the phenomenon of ''fluorescence'' from fluorite, in 1852.
Many samples of fluorite exhibit fluorescence under ultraviolet light, a property that takes its name from fluorite. Many minerals, as well as other substances, fluoresce. Fluorescence involves the elevation of electron energy levels by quanta of ultraviolet light, followed by the progressive falling back of the electrons into their previous energy state, releasing quanta of visible light in the process. In fluorite, the visible light emitted is most commonly blue, but red, purple, yellow, green, and white also occur. The fluorescence of fluorite may be due to mineral impurities, such as yttrium and ytterbium, or organic matter, such as volatile hydrocarbons in the crystal lattice. In particular, the blue fluorescence seen in fluorites from certain parts of Great Britain responsible for the naming of the phenomenon of fluorescence itself, has been attributed to the presence of inclusions of divalent europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
in the crystal. Natural samples containing rare earth impurities such as erbium have also been observed to display upconversion fluorescence, in which infrared light stimulates emission of visible light, a phenomenon usually only reported in synthetic materials.
One fluorescent variety of fluorite is chlorophane
Chlorophane, also sometimes known as pyroemerald, cobra stone, and pyrosmaragd, is a rare variety of the mineral fluorite with the unusual combined properties of thermoluminescence, thermophosphoresence, triboluminescence, and fluorescence: it w ...
, which is reddish or purple in color and fluoresces brightly in emerald green when heated (thermoluminescence
Thermoluminescence is a form of luminescence that is exhibited by certain crystalline materials, such as some minerals, when previously absorbed energy from electromagnetic radiation or other ionizing radiation is re-emitted as light upon h ...
), or when illuminated with ultraviolet light.
The color of visible light emitted when a sample of fluorite is fluorescing depends on where the original specimen was collected; different impurities having been included in the crystal lattice in different places. Neither does all fluorite fluoresce equally brightly, even from the same locality. Therefore, ultraviolet light is not a reliable tool for the identification of specimens, nor for quantifying the mineral in mixtures. For example, among British fluorites, those from Northumberland, County Durham
County Durham ( ), officially simply Durham,UK General Acts 1997 c. 23Lieutenancies Act 1997 Schedule 1(3). From legislation.gov.uk, retrieved 6 April 2022. is a ceremonial county in North East England.North East Assembly �About North East E ...
, and eastern Cumbria are the most consistently fluorescent, whereas fluorite from Yorkshire, Derbyshire, and Cornwall, if they fluoresce at all, are generally only feebly fluorescent.
Fluorite also exhibits the property of thermoluminescence
Thermoluminescence is a form of luminescence that is exhibited by certain crystalline materials, such as some minerals, when previously absorbed energy from electromagnetic radiation or other ionizing radiation is re-emitted as light upon h ...
.
Color
Fluorite is allochromatic, meaning that it can be tinted with elemental impurities. Fluorite comes in a wide range of colors and has consequently been dubbed "the most colorful mineral in the world". Every color of the rainbow in various shades is represented by fluorite samples, along with white, black, and clear crystals. The most common colors are purple, blue, green, yellow, or colorless. Less common are pink, red, white, brown, and black. Color zoning or banding is commonly present. The color of the fluorite is determined by factors including impurities, exposure to radiation, and the absence of voids of thecolor centers
An F center or Farbe center (from the original German ''Farbzentrum'', where ''Farbe'' means ''color'' and ''zentrum'' means center) is a type of crystallographic defect in which an anionic vacancy in a crystal lattice is occupied by one or more un ...
.
Uses
Source of fluorine and fluoride
Fluorite is a major source ofhydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
, a commodity chemical used to produce a wide range of materials. Hydrogen fluoride is liberated from the mineral by the action of concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
:
:CaF2( s) + H2SO4 → CaSO4(s) + 2 HF( g)
The resulting HF is converted into fluorine, fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often has distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Fluorocarbons and their derivatives are commerci ...
s, and diverse fluoride materials. As of the late 1990s, five billion kilograms were mined annually.
There are three principal types of industrial use for natural fluorite, commonly referred to as "fluorspar" in these industries, corresponding to different grades of purity. Metallurgical grade fluorite (60–85% CaF2), the lowest of the three grades, has traditionally been used as a flux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications to physics. For transport ph ...
to lower the melting point of raw materials in steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
production to aid the removal of impurities, and later in the production of aluminium. Ceramic grade fluorite (85–95% CaF2) is used in the manufacture of opalescent glass, enamels, and cooking utensils. The highest grade, "acid grade fluorite" (97% or more CaF2), accounts for about 95% of fluorite consumption in the US where it is used to make hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
and hydrofluoric acid
Hydrofluoric acid is a Solution (chemistry), solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly Corrosive substance, corrosive. It is used to make most fluorine-containing compounds; examples include th ...
by reacting the fluorite with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
.
Internationally, acid-grade fluorite is also used in the production of AlF3 and cryolite (Na3AlF6), which are the main fluorine compounds used in aluminium smelting. Alumina is dissolved in a bath that consists primarily of molten Na3AlF6, AlF3, and fluorite (CaF2) to allow electrolytic recovery of aluminium. Fluorine losses are replaced entirely by the addition of AlF3, the majority of which react with excess sodium from the alumina to form Na3AlF6.Miller, M. MichaelFluorspar
USGS 2009 Minerals Yearbook
Niche uses

Lapidary uses
Natural fluorite mineral has ornamental and lapidary uses. Fluorite may be drilled into beads and used in jewelry, although due to its relative softness it is not widely used as a semiprecious stone. It is also used for ornamental carvings, with expert carvings taking advantage of the stone's zonation.Optics
In the laboratory, calcium fluoride is commonly used as a window material for both infrared and ultraviolet wavelengths, since it is transparent in these regions (about 0.15 µm to 9 µm) and exhibits an extremely low change in refractive index with wavelength. Furthermore, the material is attacked by few reagents. At wavelengths as short as 157 nm, a common wavelength used for semiconductor stepper manufacture forintegrated circuit
An integrated circuit or monolithic integrated circuit (also referred to as an IC, a chip, or a microchip) is a set of electronic circuits on one small flat piece (or "chip") of semiconductor material, usually silicon. Large numbers of tiny ...
lithography, the refractive index of calcium fluoride shows some non-linearity at high power densities, which has inhibited its use for this purpose. In the early years of the 21st century, the stepper market for calcium fluoride collapsed, and many large manufacturing facilities have been closed. Canon and other manufacturers have used synthetically grown crystals of calcium fluoride components in lenses to aid apochromatic design, and to reduce light dispersion. This use has largely been superseded by newer glasses and computer-aided design. As an infrared optical material, calcium fluoride is widely available and was sometimes known by the Eastman Kodak
The Eastman Kodak Company (referred to simply as Kodak ) is an American public company that produces various products related to its historic basis in analogue photography. The company is headquartered in Rochester, New York, and is incorpor ...
trademarked name "Irtran-3", although this designation is obsolete.
Fluorite should not be confused with fluoro-crown (or fluorine crown) glass, a type of low-dispersion glass that has special optical properties approaching fluorite. True fluorite is not a glass but a crystalline material. Lenses or optical groups made using this low dispersion glass as one or more elements exhibit less chromatic aberration than those utilizing conventional, less expensive crown glass and flint glass elements to make an achromatic lens. Optical groups employ a combination of different types of glass; each type of glass refracts light in a different way. By using combinations of different types of glass, lens manufacturers are able to cancel out or significantly reduce unwanted characteristics; chromatic aberration being the most important. The best of such lens designs are often called apochromatic (see above). Fluoro-crown glass (such as Schott FK51) usually in combination with an appropriate "flint" glass (such as Schott KzFSN 2) can give very high performance in telescope objective lenses, as well as microscope objectives, and camera telephoto lenses. Fluorite elements are similarly paired with complementary "flint" elements (such as Schott LaK 10). The refractive qualities or fluorite and of certain flint elements provide a lower and more uniform dispersion across the spectrum of visible light, thereby keeping colors focused more closely together. Lenses made with fluorite are superior to fluoro-crown based lenses, at least for doublet telescope objectives; but are more difficult to produce and more costly.
The use of fluorite for prisms and lenses was studied and promoted by Victor Schumann near the end of the 19th century. Naturally occurring fluorite crystals without optical defects were only large enough to produce microscope objectives.
With the advent of synthetically grown fluorite crystals in the 1950s - 60s, it could be used instead of glass in some high-performance optical telescope and camera lens
A camera lens (also known as photographic lens or photographic objective) is an optical lens or assembly of lenses used in conjunction with a camera body and mechanism to make images of objects either on photographic film or on other media capab ...
elements. In telescopes, fluorite elements allow high-resolution images of astronomical objects at high magnification
Magnification is the process of enlarging the apparent size, not physical size, of something. This enlargement is quantified by a calculated number also called "magnification". When this number is less than one, it refers to a reduction in siz ...
s. Canon Inc. produces synthetic fluorite crystals that are used in their better telephoto lenses. The use of fluorite for telescope lenses has declined since the 1990s, as newer designs using fluoro-crown glass, including triplets, have offered comparable performance at lower prices. Fluorite and various combinations of fluoride compounds can be made into synthetic crystals which have applications in lasers and special optics for UV and infrared.
Exposure tools for the semiconductor industry make use of fluorite optical elements for ultraviolet light at wavelengths of about 157 nanometer
330px, Different lengths as in respect to the molecular scale.
The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re ...
s. Fluorite has a uniquely high transparency at this wavelength. Fluorite objective lenses are manufactured by the larger microscope firms (Nikon, Olympus
Olympus or Olympos ( grc, Ὄλυμπος, link=no) may refer to:
Mountains
In antiquity
Greece
* Mount Olympus in Thessaly, northern Greece, the home of the twelve gods of Olympus in Greek mythology
* Mount Olympus (Lesvos), located in Les ...
, Carl Zeiss and Leica). Their transparence to ultraviolet light enables them to be used for fluorescence microscopy. The fluorite also serves to correct optical aberration
In optics, aberration is a property of optical systems, such as lenses, that causes light to be spread out over some region of space rather than focused to a point. Aberrations cause the image formed by a lens to be blurred or distorted, with th ...
s in these lenses. Nikon has previously manufactured at least one fluorite and synthetic quartz element camera lens (105 mm f/4.5 UV) for the production of ultraviolet images. Konica produced a fluorite lens for their SLR cameras – the Hexanon 300 mm f/6.3.
Source of fluorine gas in nature
In 2012, the first source of naturally occurring fluorine gas was found in fluorite mines in Bavaria, Germany. It was previously thought that fluorine gas did not occur naturally because it is so reactive, and would rapidly react with other chemicals. Fluorite is normally colorless, but some varied forms found nearby look black, and are known as 'fetid fluorite' or antozonite. The minerals, containing small amounts of uranium and its daughter products, release radiation sufficiently energetic to induce oxidation of fluoride anions within the structure, to fluorine that becomes trapped inside the mineral. The color of fetid fluorite is predominantly due to the calcium atoms remaining. Solid-state fluorine-19 NMR carried out on the gas contained in the antozonite, revealed a peak at 425 ppm, which is consistent with F2.Withers, Neil (1 July 2012Fluorine finally found in nature , Chemistry World
Rsc.org.
Images
See also
* List of countries by fluorite production * List of minerals * Magnesium fluoride – also used in UV opticsReferences
External links
Educational article about the different colors of fluorites crystals from Asturias, Spain
an
Crawford Cup
related Roman cups at British Museum {{Authority control Cubic minerals Minerals in space group 225 Evaporite Fluorine minerals Luminescent minerals Industrial minerals Symbols of Illinois