Flame Conductivity on:
[Wikipedia]
[Google]
[Amazon]
 A flame (from Latin ''
A flame (from Latin ''
 Color and temperature of a flame are dependent on the type of fuel involved in the combustion, as, for example, when a lighter is held to a
Color and temperature of a flame are dependent on the type of fuel involved in the combustion, as, for example, when a lighter is held to a
 Flame color depends on several factors, the most important typically being black-body radiation and
Flame color depends on several factors, the most important typically being black-body radiation and  In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from incandescence of very fine soot particles that are produced in the flame. Also,
In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from incandescence of very fine soot particles that are produced in the flame. Also,
 When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of
When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of
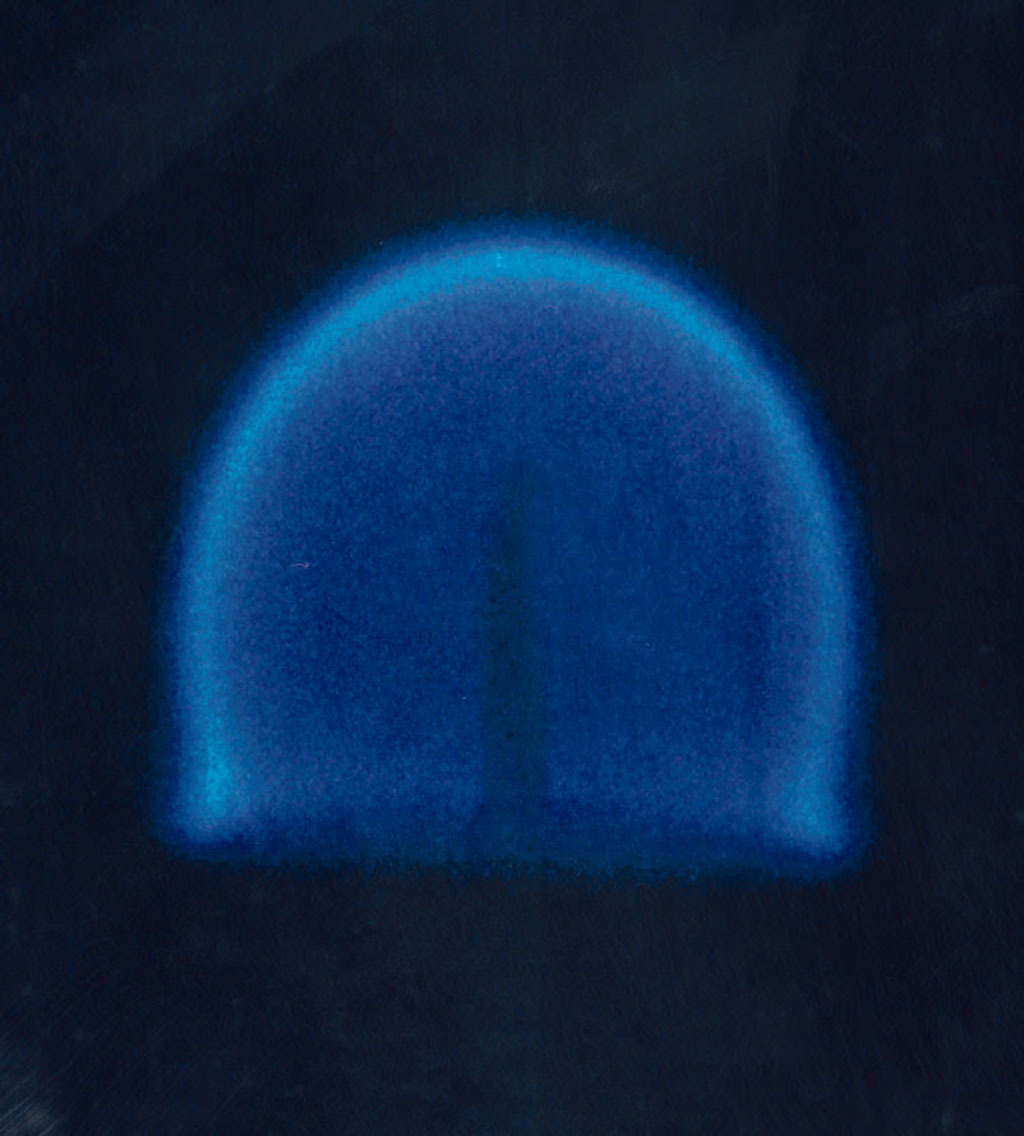 In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on
In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on
A candle flame strongly influenced and moved about by an electric field due to the flame having ions.
Ultra-Low Emissions Low-Swirl Burner
7 Shades of Fire
* {{Authority control Fire lez:ЦӀай
 A flame (from Latin ''
A flame (from Latin ''flamma
Flamma (lit. The Flame) was a Syrian gladiator under the Roman Empire during the reign of Hadrian. He was one of the most famous and successful of his time.
History
How Flamma ended up as a gladiator is unknown. He may have been a revolution ...
'') is the visible, gaseous part of a fire
Fire is the rapid oxidation of a material (the fuel) in the exothermic chemical process of combustion, releasing heat, light, and various reaction products.
At a certain point in the combustion reaction, called the ignition point, flames a ...
. It is caused by a highly exothermic chemical reaction taking place in a thin zone. When flames are hot enough to have ionize
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule ...
d gaseous components of sufficient density they are then considered plasma.
Mechanism
candle
A candle is an ignitable wick embedded in wax, or another flammable solid substance such as tallow, that provides light, and in some cases, a fragrance. A candle can also provide heat or a method of keeping time.
A person who makes candle ...
. The applied heat causes the fuel molecules in the candle wax
A candle is an ignitable wick embedded in wax, or another flammable solid substance such as tallow, that provides light, and in some cases, a fragrance. A candle can also provide heat or a method of keeping time.
A person who makes candles ...
to vaporize (if this process happens in inert atmosphere without oxidizer
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
, it is called pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''py ...
). In this state they can then readily react with oxygen in the air, which gives off enough heat in the subsequent exothermic reaction to vaporize yet more fuel, thus sustaining a consistent flame. The high temperature of the flame causes the vaporized fuel molecules to decompose
Decomposition or rot is the process by which dead organic substances are broken down into simpler organic or inorganic matter such as carbon dioxide, water, simple sugars and mineral salts. The process is a part of the nutrient cycle and is ...
, forming various incomplete combustion products and free radicals, and these products then react with each other and with the oxidizer
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxid ...
involved in the reaction of the following flame (fire). One may investigate all the different parts of the flame from a candle with a cold metal spoon: Higher parts are water vapor, the result of combustion; yellow parts in the middle are soot; down just next to the candle wick is unburned wax. Goldsmiths use higher parts of a flame with a metallic blow-pipe for melting gold and silver. Sufficient energy in the flame will excite the electrons in some of the transient reaction intermediates such as the methylidyne radical
Methylidyne, or (unsubstituted) carbyne, is an organic compound whose molecule consists of a single hydrogen atom bonded to a carbon atom. It is the parent compound of the carbynes, which can be seen as obtained from it by substitution of other ...
(CH) and diatomic carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed thro ...
(C2), which results in the emission of visible light as these substances release their excess energy (see spectrum below for an explanation of which specific radical species produce which specific colors). As the combustion temperature of a flame increases (if the flame contains small particles of unburnt carbon or other material), so does the average energy of the electromagnetic radiation given off by the flame (see Black body
A black body or blackbody is an idealized physical body that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. The name "black body" is given because it absorbs all colors of light. A black body ...
).
Other oxidizers besides oxygen can be used to produce a flame. Hydrogen burning in chlorine produces a flame and in the process emits gaseous hydrogen chloride
The compound hydrogen chloride has the chemical formula and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride ga ...
(HCl) as the combustion product. Another of many possible chemical combinations is hydrazine and nitrogen tetroxide
Dinitrogen tetroxide, commonly referred to as nitrogen tetroxide (NTO), and occasionally (usually among ex-USSR/Russia rocket engineers) as amyl, is the chemical compound N2O4. It is a useful reagent in chemical synthesis. It forms an equilibrium ...
which is hypergolic
A hypergolic propellant is a rocket propellant combination used in a rocket engine, whose components spontaneously ignite when they come into contact with each other.
The two propellant components usually consist of a fuel and an oxidizer. T ...
and commonly used in rocket engines. Fluoropolymers can be used to supply fluorine as an oxidizer of metallic fuels, e.g. in the magnesium/teflon/viton composition.
The chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in ...
occurring in the flame are very complex and typically involve a large number of chemical reactions and intermediate species, most of them radicals. For instance, a well-known chemical kinetics scheme, GRI-Mech, uses 53 species and 325 elementary reactions to describe combustion of biogas
Biogas is a mixture of gases, primarily consisting of methane, carbon dioxide and hydrogen sulphide, produced from raw materials such as agricultural waste, manure, municipal waste, plant material, sewage, green waste and food waste. It is a ...
.
There are different methods of distributing the required components of combustion to a flame. In a diffusion flame
In combustion, a diffusion flame is a flame in which the oxidizer and fuel are separated before burning. Contrary to its name, a diffusion flame involves both diffusion and convection processes. The name diffusion flame was first suggested by S. ...
, oxygen and fuel diffuse into each other; the flame occurs where they meet. In a premixed flame
A premixed flame is a flame formed under certain conditions during the combustion of a premixed charge (also called pre-mixture) of fuel and oxidiser. Since the fuel and oxidiser—the key chemical reactants of combustion—are available througho ...
, the oxygen and fuel are premixed beforehand, which results in a different type of flame. Candle flames (a diffusion flame) operate through evaporation of the fuel which rises in a laminar flow of hot gas which then mixes with surrounding oxygen and combusts.
Color
 Flame color depends on several factors, the most important typically being black-body radiation and
Flame color depends on several factors, the most important typically being black-body radiation and spectral band
Spectral bands are parts of the electromagnetic spectrum of specific wavelengths, which can be filtered by a standard filter. In nuclear physics, spectral bands are referred to the emission of polyatomic systems, including condensed materials, larg ...
emission, with both spectral line
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to iden ...
emission and spectral line absorption playing smaller roles. In the most common type of flame, hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
flames, the most important factor determining color is oxygen supply and the extent of fuel-oxygen pre-mixing, which determines the rate of combustion and thus the temperature and reaction paths, thereby producing different color hues.
 In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from incandescence of very fine soot particles that are produced in the flame. Also,
In a laboratory under normal gravity conditions and with a closed air inlet, a Bunsen burner burns with yellow flame (also called a safety flame) with a peak temperature of about . The yellow arises from incandescence of very fine soot particles that are produced in the flame. Also, carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
is produced, and the flame tends to take oxygen from the surfaces it touches. When the air inlet is opened, less soot and carbon monoxide are produced. When enough air is supplied, no soot or carbon monoxide is produced and the flame becomes blue. (Most of this blue had previously been obscured by the bright yellow emissions.) The spectrum of a premixed (complete combustion) butane flame on the right shows that the blue color arises specifically due to emission of excited molecular radicals in the flame, which emit most of their light well below ≈565 nanometers in the blue and green regions of the visible spectrum.
The colder part of a diffusion (incomplete combustion) flame will be red, transitioning to orange, yellow, and white as the temperature increases as evidenced by changes in the black-body radiation spectrum. For a given flame's region, the closer to white on this scale, the hotter that section of the flame is. The transitions are often apparent in fires, in which the color emitted closest to the fuel is white, with an orange section above it, and reddish flames the highest of all. A blue-colored flame only emerges when the amount of soot decreases and the blue emissions from excited molecular radicals become dominant, though the blue can often be seen near the base of candles where airborne soot is less concentrated.
Specific colors can be imparted to the flame by introduction of excitable species with bright emission spectrum lines. In analytical chemistry, this effect is used in flame test
A flame test is an analytical procedure used in chemistry to detect the presence of certain elements, primarily metal ions, based on each element's characteristic flame emission spectrum (which may be affected by the presence of chloride io ...
s (or flame emission spectroscopy) to determine presence of some metal ions. In pyrotechnics, the pyrotechnic colorant
A pyrotechnic colorant is a chemical compound which causes a flame to burn with a particular color. These are used to create the colors in pyrotechnic compositions like fireworks and colored fires. The color-producing species are usually creat ...
s are used to produce brightly colored fireworks.
Temperature
 When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of
When looking at a flame's temperature there are many factors which can change or apply. An important one is that a flame's color does not necessarily determine a temperature comparison because black-body radiation is not the only thing that produces or determines the color seen; therefore it is only an estimation of temperature. Other factors that determine its temperature are:
* In fires (particularly house fires), the cooler flames are often red and produce the most smoke. Here the red color compared to typical yellow color of the flames suggests that the temperature is lower. This is because there is a lack of oxygen in the room and therefore there is incomplete combustion and the flame temperature is low, often just . This means that a lot of carbon monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simple ...
is formed (which is a flammable gas) which is when there is greatest risk of backdraft
A backdraft ( North American English) or backdraught (British English) is the abrupt burning of superheated gasses in a fire, caused when oxygen rapidly enters a hot, oxygen-depleted environment; for example, when a window or door to an enclosed ...
. When this occurs, combustible gases at or above the flash point of spontaneous combustion are exposed to oxygen, carbon monoxide and superheated hydrocarbons combust, and temporary temperatures of up to occur.
Common flame temperatures
This is a rough guide to flame temperatures for various common substances (in air at 1 atm. pressure):Highest temperature
Dicyanoacetylene
Dicyanoacetylene, also called carbon subnitride or but-2-ynedinitrile (IUPAC), is a compound of carbon and nitrogen with chemical formula . It has a linear molecular structure, (often abbreviated as ), with alternating triple and single covalen ...
, a compound of carbon and nitrogen with chemical formula C4N2 burns in oxygen with a bright blue-white flame at a temperature of , and at up to in ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
. This high flame temperature is partially due to the absence of hydrogen in the fuel (dicyanoacetylene is not a hydrocarbon) thus there is no water among the combustion products.
Cyanogen
Cyanogen is the chemical compound with the formula ( C N)2. It is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups – analogous to diatomic halogen molecu ...
, with the formula (CN)2, produces the second-hottest-known natural flame with a temperature of over when it burns in oxygen.
Cool flames
At temperatures as low as , fuel-air mixtures can react chemically and produce very weak flames called cool flames. The phenomenon was discovered byHumphry Davy
Sir Humphry Davy, 1st Baronet, (17 December 177829 May 1829) was a British chemist and inventor who invented the Davy lamp and a very early form of arc lamp. He is also remembered for isolating, by using electricity, several elements for t ...
in 1817. The process depends on a fine balance of temperature and concentration of the reacting mixture, and if conditions are right it can initiate without any external ignition source. Cyclical variations in the balance of chemicals, particularly of intermediate products in the reaction, give oscillations in the flame, with a typical temperature variation of about , or between "cool" and full ignition. Sometimes the variation can lead to an explosion.
In microgravity
 In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on
In the year 2000, experiments by NASA confirmed that gravity plays an indirect role in flame formation and composition. The common distribution of a flame under normal gravity conditions depends on convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the conve ...
, as soot tends to rise to the top of a flame (such as in a candle in normal gravity conditions), making it yellow. In microgravity or zero gravity
Weightlessness is the complete or near-complete absence of the sensation of weight. It is also termed zero gravity, zero G-force, or zero-G.
Weight is a measurement of the force on an object at rest in a relatively strong gravitational f ...
environment, such as in orbit, natural convection no longer occurs and the flame becomes spherical, with a tendency to become bluer and more efficient. There are several possible explanations for this difference, of which the most likely is the hypothesis that the temperature is sufficiently evenly distributed that soot is not formed and complete combustion occurs. Experiments by NASA
The National Aeronautics and Space Administration (NASA ) is an independent agencies of the United States government, independent agency of the US federal government responsible for the civil List of government space agencies, space program ...
reveal that diffusion flames in microgravity allow more soot to be completely oxidized after they are produced than do diffusion flames on Earth, because of a series of mechanisms that behave differently in microgravity when compared to normal gravity conditions. These discoveries have potential applications in applied science and private industry, especially concerning fuel efficiency.
Thermonuclear flames
Flames do not need to be driven only by chemical energy release. In stars, subsonic burning fronts driven by burning light nuclei (like carbon or helium) to heavy nuclei (up to iron group) propagate as flames. This is important in some models ofType Ia supernovae
A Type Ia supernova (read: "type one-A") is a type of supernova that occurs in binary systems (two stars orbiting one another) in which one of the stars is a white dwarf. The other star can be anything from a giant star to an even smaller white ...
. In thermonuclear flames, thermal conduction dominates over species diffusion, so the flame speed and thickness is determined by the thermonuclear energy release and thermal conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa.
Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal ...
(often in the form of degenerate electrons).
See also
*Flame detector
A flame detector is a sensor designed to detect and respond to the presence of a flame or fire, allowing flame detection. Responses to a detected flame depend on the installation, but can include sounding an alarm, deactivating a fuel line (such as ...
* International Flame Research Foundation
The International Flame Research Foundation – IFRF is a non-profit research association and network created in 1948 in IJmuiden (Netherlands), established in Livorno (Italy) between 2005 and 2016 ''(Fondazione Internazionale per la Ricerca Sulla ...
* Oxidizing and reducing flames
In pyrology, flames are affected by the fuel introduced and the oxygen available. A flame with a good oxygen-fuel ratio is called a neutral flame. The color of the flame is semi-transparent purple or blue. This flame is optimal for all intentions, ...
* The Combustion Institute
The Combustion Institute is an educational non-profit, international, scientific and engineering society whose purpose is to promote research in combustion science. The institute was established in 1954, and its headquarters are in Pittsburgh, Pen ...
References
External links
A candle flame strongly influenced and moved about by an electric field due to the flame having ions.
Ultra-Low Emissions Low-Swirl Burner
7 Shades of Fire
* {{Authority control Fire lez:ЦӀай