Fischer Indole Synthesis on:
[Wikipedia]
[Google]
[Amazon]
The Fischer indole synthesis is a  This reaction can be catalyzed by Brønsted acids such as
This reaction can be catalyzed by Brønsted acids such as
 Isotopic labelling studies show that the aryl nitrogen (N1) of the starting phenylhydrazine is incorporated into the resulting indole.
Isotopic labelling studies show that the aryl nitrogen (N1) of the starting phenylhydrazine is incorporated into the resulting indole.

chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
that produces the aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
indole
Indole is an aromatic heterocyclic organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other c ...
from a (substituted) phenylhydrazine
Phenylhydrazine is the chemical compound with the formula . It is often abbreviated as . It is also found in edible mushrooms.
Properties
Phenylhydrazine forms monoclinic prisms that melt to an oil around room temperature which may turn yellow ...
and an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of draw ...
. Today antimigraine
Antimigraine drugs are medications intended to reduce the effects or intensity of migraine headache. They include drugs for the treatment of acute migraine symptoms as well as drugs for the prevention of migraine attacks.
Treatment of acute sy ...
drugs of the triptan
Triptans are a family of tryptamine-based drugs used as abortive medication in the treatment of migraines and cluster headaches. This drug class was first commercially introduced in the 1990s. While effective at treating individual headaches, t ...
class are often synthesized by this method.
HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a spe ...
, H2SO4, polyphosphoric acid
A phosphoric acid, in the general sense, is a phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is bonded to four oxygen (O) atoms, one of them through a double bond, arranged as the corners of a tetrahedron. ...
and p-toluenesulfonic acid
''p''-Toluenesulfonic acid (PTSA or ''p''TsOH) or tosylic acid (TsOH) is an organic compound with the formula CH3 C6H4 SO3H. It is a white extremely hygroscopic solid that is soluble in water, alcohols, and other polar organic solvents. The CH3C ...
or Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s such as boron trifluoride, zinc chloride
Zinc chloride is the name of inorganic chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. This salt is hygroscopic and e ...
, iron chloride Iron chloride may refer to:
* Iron(II) chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are o ...
, and aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contam ...
.
Several reviews have been published.
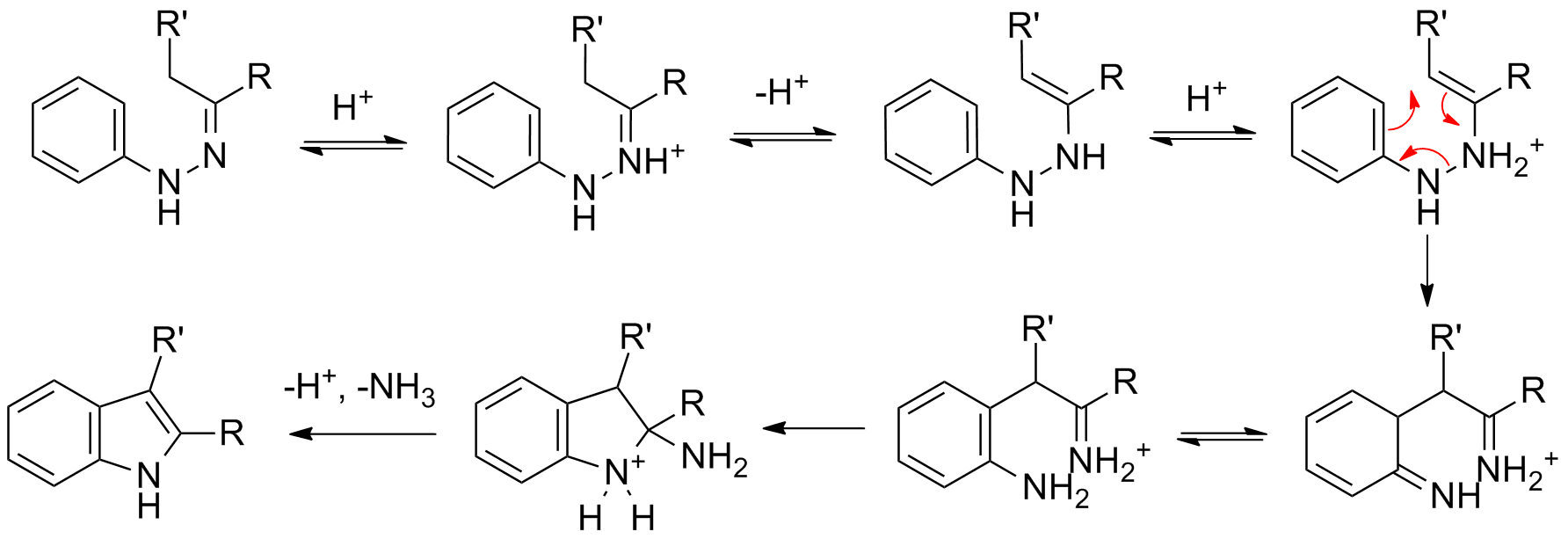
Reaction mechanism
The reaction of a (substituted) phenylhydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
with a carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
(aldehyde or ketone) initially forms a phenylhydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. ...
which isomerizes to the respective enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
:
The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and t ...
(or 'ene-hydrazine'). After protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
, a cyclic ,3sigmatropic rearrangement occurs producing an imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
. The resulting imine forms a cyclic aminoacetal (or ''aminal''), which under acid catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
eliminates NH3, resulting in the energetically favorable aromatic indole.
 Isotopic labelling studies show that the aryl nitrogen (N1) of the starting phenylhydrazine is incorporated into the resulting indole.
Isotopic labelling studies show that the aryl nitrogen (N1) of the starting phenylhydrazine is incorporated into the resulting indole.
Buchwald modification
Via apalladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
-catalyzed reaction, the Fischer indole synthesis can be effected by cross-coupling aryl bromides and hydrazones. This result supports the previously proposed intermediacy as hydrazone intermediates in the classical Fischer indole synthesis. These ''N''-arylhydrazones undergo exchange with other ketones, expanding the scope of this method.

Application
*Indometacin
Indometacin, also known as indomethacin, is a nonsteroidal anti-inflammatory drug (NSAID) commonly used as a prescription medication to reduce fever, pain, stiffness, and swelling from inflammation. It works by inhibiting the production of pros ...
preparation.
*Triptan
Triptans are a family of tryptamine-based drugs used as abortive medication in the treatment of migraines and cluster headaches. This drug class was first commercially introduced in the 1990s. While effective at treating individual headaches, t ...
synthesis
*Iprindole
Iprindole, sold under the brand names Prondol, Galatur, and Tertran, is an atypical tricyclic antidepressant (TCA) that has been used in the United Kingdom and Ireland for the treatment of depression but appears to no longer be marketed. It was ...
synthesis (phenylhydrazine
Phenylhydrazine is the chemical compound with the formula . It is often abbreviated as . It is also found in edible mushrooms.
Properties
Phenylhydrazine forms monoclinic prisms that melt to an oil around room temperature which may turn yellow ...
+ suberone → 2,3-Cycloheptenoindole).
See also
*Bartoli indole synthesis
The Bartoli indole synthesis (also called the Bartoli reaction) is the chemical reaction of ortho-substituted nitroarenes and nitrosoarenes with vinyl Grignard reagents to form substituted indoles.
The reaction is often unsuccessful without sub ...
* Japp–Klingemann indole synthesis
*Leimgruber–Batcho indole synthesis The Leimgruber–Batcho indole synthesis is a series of organic reactions that produce indoles from o-nitrotoluenes 1. The first step is the formation of an enamine 2 using N,N-dimethylformamide dimethyl acetal and pyrrolidine. The desired indole ...
*Larock indole synthesis
The Larock indole synthesis is a heteroannulation reaction that uses palladium as a catalyst to synthesize indoles from an ortho-iodoaniline and a disubstituted alkyne. It is also known as Larock heteroannulation. The reaction is extremely versatil ...
Related reactions
*Madelung synthesis
The Madelung synthesis is a chemical reaction that produces (substituted or unsubstituted) indoles by the intramolecular cyclization of N-phenylamides using strong base at high temperature. The Madelung synthesis was reported in 1912 by Walter Ma ...
* Reissert synthesis
* Gassman synthesis
* Nenitzescu synthesis
References
{{Reflist Indole forming reactions Name reactions Emil Fischer