|
Phenylhydrazine
Phenylhydrazine is the chemical compound with the formula . It is often abbreviated as . It is also found in edible mushrooms. Properties Phenylhydrazine forms monoclinic prisms that melt to an oil around room temperature which may turn yellow to dark red upon exposure to air. Phenylhydrazine is miscible with ethanol, diethyl ether, chloroform and benzene. It is sparingly soluble in water. Preparation Phenylhydrazine is prepared by oxidizing aniline with sodium nitrite in the presence of hydrogen chloride to form the diazonium salt, which is subsequently reduced using sodium sulfite in the presence of sodium hydroxide to form the final product.''Merck Index of Chemicals and Drugs, 9th ed.'' monograph 7098 History Phenylhydrazine was the first hydrazine derivative characterized, reported by Hermann Emil Fischer in 1875. He prepared it by reduction of a phenyl diazonium salt using sulfite salts. Fischer used phenylhydrazine to characterize sugars via formation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazines
Hydrazines (R2N−NR2) are a class of chemical compounds with two nitrogen atoms linked via a covalent bond and which carry from one up to four alkyl or aryl substituents. Hydrazines can be considered as derivatives of the inorganic hydrazine (H2N−NH2), in which one or more hydrogen atoms have been replaced by hydrocarbon groups. Production * 1,1-Dimethylhydrazine is produced by the reduction of ''N''-nitrosodimethylamine.Siegfried Hauptmann: ''Organische Chemie'', 2. durchgesehene Auflage, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, S. 522–523, . * The reduction of benzenediazonium chloride with tin(II) chloride and hydrochloric acid provides phenylhydrazine. * 2,4-Dinitrophenylhydrazine is produced by the reaction of 1-chloro-2,4-dinitrobenzene with hydrazine. * Tetraphenylhydrazine is formed by the oxidation of diphenylamine with potassium permanganate in acetone. Classification Hydrazines can be divided into three groups according to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fischer Indole Synthesis
The Fischer indole synthesis is a chemical reaction that produces the aromatic Heterocyclic compound, heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today migraine, antimigraine drugs of the triptan class are often synthesized by this method. This reaction can be catalyzed by Brønsted acids such as HCl, sulfuric acid, H2SO4, polyphosphoric acid and p-toluenesulfonic acid or Lewis acids such as boron trifluoride, zinc chloride, iron chloride, and aluminium chloride. Several reviews have been published. Reaction mechanism The reaction of a (substituted) phenylhydrazine with a carbonyl (aldehyde or ketone) initially forms a phenylhydrazone which isomerization, isomerizes to the respective enamine (or 'ene-hydrazine'). After protonation, a cyclic sigmatropic rearrangement, [3,3]-sigmatropic rearrangement occurs producing an imine. The resulting imine forms a cyclic amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms. He also hypothesized lock and key mechanism of enzyme action. He never used his first given name, and was known throughout his life simply as Emil Fischer. Early years and career Fischer was born in Euskirchen, near Cologne, the son of Laurenz Fischer, a businessman, and his wife Julie Poensgen. After graduating he wished to study natural sciences, but his father compelled him to work in the family business until determining that his son was unsuitable. Fischer then attended the University of Bonn in 1871, but switched to the University of Strasbourg in 1872. He earned his doctorate in 1874 under Adolf von Baeyer with his study of phthaleins, and was appointed to a position at the university. After eight yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osazone
Osazones are a class of carbohydrate derivatives found in organic chemistry formed when Reducing sugar, reducing sugars are reacted with excess of phenylhydrazine at boiling temperatures. Formation Osazone formation was developed by Emil Fischer, who used the reaction as a test to identify monosaccharides. The formation of a pair of hydrazone functionalities involves both oxidation and condensation reactions. Since the reaction requires a free carbonyl group, only "reducing sugars" participate. Sucrose, which is nonreducing, does not form an osazone. : : Appearance Osazones are highly coloured and crystalline compounds. Osazones are readily distinguished. *Maltosazone (from maltose) forms petal-shaped crystals. *Lactosazone (from lactose) forms powder puff-shaped crystals. *Galactosazone (from galactose) forms rhombic-plate shaped crystals. *Glucosazone (from glucose, fructose or mannose) forms broomstick or needle-shaped crystals. Historic references * * * References< ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hermann Emil Fischer
Hermann Emil Louis Fischer (; 9 October 1852 – 15 July 1919) was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms. He also hypothesized lock and key mechanism of enzyme action. He never used his first given name, and was known throughout his life simply as Emil Fischer. Early years and career Fischer was born in Euskirchen, near Cologne, the son of Laurenz Fischer, a businessman, and his wife Julie Poensgen. After graduating he wished to study natural sciences, but his father compelled him to work in the family business until determining that his son was unsuitable. Fischer then attended the University of Bonn in 1871, but switched to the University of Strasbourg in 1872. He earned his doctorate in 1874 under Adolf von Baeyer with his study of phthaleins, and was appointed to a position at the university. After eight yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylhydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2-Orbital hybridisation, hybridized. The aldehyde group is somewhat polar molecule, polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (or the sulfate(IV) ion, from its correct systematic name), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoamine Oxidase Inhibitors
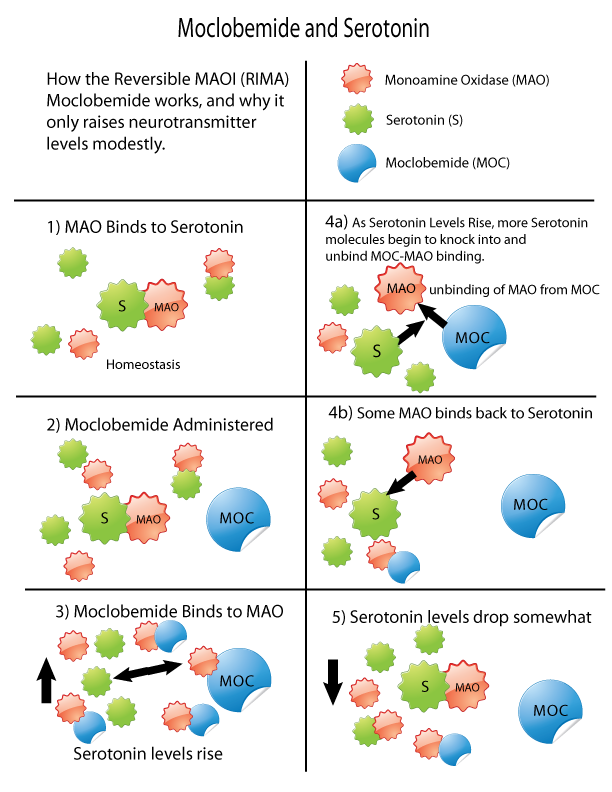
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphobia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver Damage
Liver disease, or hepatic disease, is any of many diseases of the liver. If long-lasting it is termed chronic liver disease. Although the diseases differ in detail, liver diseases often have features in common. Signs and symptoms Some of the signs and symptoms of a liver disease are the following: * Jaundice * Confusion and altered consciousness caused by hepatic encephalopathy. * Thrombocytopenia and coagulopathy. * Risk of bleeding symptoms particularly taking place in gastrointestinal tract Liver diseases File:Ground glass hepatocytes high mag cropped 2.jpg, Ground glass hepatocytes File:Primary biliary cirrhosis intermed mag much cropping.jpg, Primary biliary cirrhosis File:Buddchiari2.PNG, Budd-chiari syndrome File:Non-alcoholic_fatty_liver_disease1.jpg, Micrograph of non-alcoholic fatty liver disease There are more than a hundred different liver diseases. Some of the most common are: * Fascioliasis, a parasitic infection of liver caused by a liver fluke of the genus ''F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |