ferromagnet on:
[Wikipedia]
[Google]
[Amazon]
 Ferromagnetism is a property of certain materials (such as
Ferromagnetism is a property of certain materials (such as
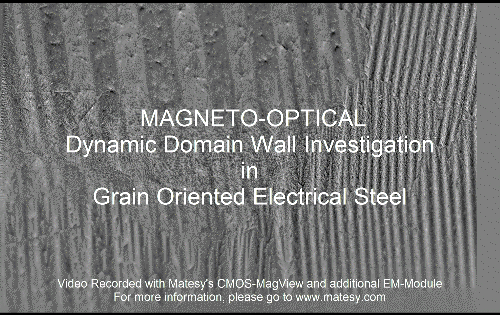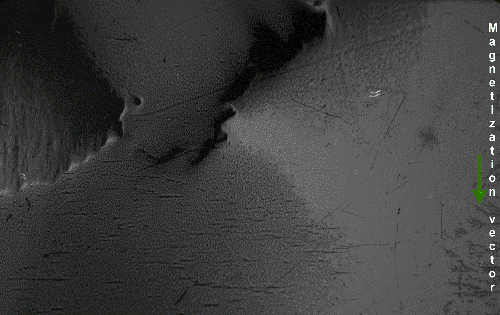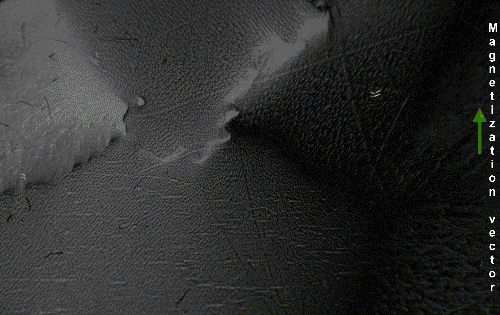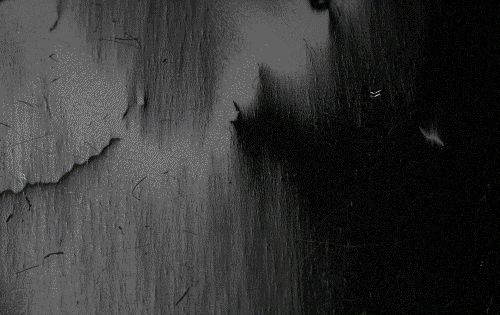
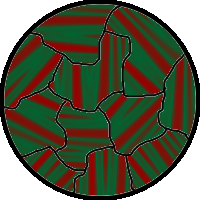 The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called '' magnetic domains''
(also known as ''Weiss domains''). Within each domain, the spins are aligned, but (if the bulk material is in its lowest energy configuration; i.e. ''unmagnetized''), the spins of separate domains point in different directions and their magnetic fields cancel out, so the object has no net large scale magnetic field.
Ferromagnetic materials spontaneously divide into magnetic domains because the '' exchange interaction'' is a short-range force, so over long distances of many atoms the tendency of the magnetic dipoles to reduce their energy by orienting in opposite directions wins out. If all the dipoles in a piece of ferromagnetic material are aligned parallel, it creates a large magnetic field extending into the space around it. This contains a lot of magnetostatic energy. The material can reduce this energy by splitting into many domains pointing in different directions, so the magnetic field is confined to small local fields in the material, reducing the volume of the field. The domains are separated by thin domain walls a number of molecules thick, in which the direction of magnetization of the dipoles rotates smoothly from one domain's direction to the other.
The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called '' magnetic domains''
(also known as ''Weiss domains''). Within each domain, the spins are aligned, but (if the bulk material is in its lowest energy configuration; i.e. ''unmagnetized''), the spins of separate domains point in different directions and their magnetic fields cancel out, so the object has no net large scale magnetic field.
Ferromagnetic materials spontaneously divide into magnetic domains because the '' exchange interaction'' is a short-range force, so over long distances of many atoms the tendency of the magnetic dipoles to reduce their energy by orienting in opposite directions wins out. If all the dipoles in a piece of ferromagnetic material are aligned parallel, it creates a large magnetic field extending into the space around it. This contains a lot of magnetostatic energy. The material can reduce this energy by splitting into many domains pointing in different directions, so the magnetic field is confined to small local fields in the material, reducing the volume of the field. The domains are separated by thin domain walls a number of molecules thick, in which the direction of magnetization of the dipoles rotates smoothly from one domain's direction to the other.
 Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the Barkhausen effect: as the magnetizing field is changed, the magnetization changes in thousands of tiny discontinuous jumps as the domain walls suddenly "snap" past defects.
This magnetization as a function of the external field is described by a hysteresis curve. Although this state of aligned domains found in a piece of magnetized ferromagnetic material is not a minimal-energy configuration, it is
Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the Barkhausen effect: as the magnetizing field is changed, the magnetization changes in thousands of tiny discontinuous jumps as the domain walls suddenly "snap" past defects.
This magnetization as a function of the external field is described by a hysteresis curve. Although this state of aligned domains found in a piece of magnetized ferromagnetic material is not a minimal-energy configuration, it is
Electromagnetism
– ch. 11, from an online textbook * Detailed nonmathematical description of ferromagnetic materials with illustrations
Magnetism: Models and Mechanisms
in E. Pavarini, E. Koch, and U. Schollwöck: Emergent Phenomena in Correlated Matter, Jülich 2013, {{Authority control Quantum phases Magnetic hysteresis Physical phenomena Ferromagnetism
 Ferromagnetism is a property of certain materials (such as
Ferromagnetism is a property of certain materials (such as iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials are the familiar metals noticeably attracted to a magnet, a consequence of their large magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an ''external'' magnetic field, and it is this temporarily induced magnetization inside a steel plate, for instance, which accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself, depends not only on the strength of the applied field, but on the so-called coercivity of that material, which varies greatly among ferromagnetic materials.
In physics
Physics is the natural science that studies matter, its fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge which rel ...
, several different types of material magnetism
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particle ...
are distinguished. Ferromagnetism (along with the similar effect ferrimagnetism) is the strongest type and is responsible for the common phenomenon of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism— paramagnetism, diamagnetism, and antiferromagnetism—but the forces are usually so weak that they can be detected only by sensitive instruments in a laboratory. An everyday example of a permanent magnet formed of a ferromagnetic material is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and a ferromagnetic material like iron has been described as "the quality of magnetism first apparent to the ancient world, and to us today".Bozorth, Richard M. ''Ferromagnetism'', first published 1951, reprinted 1993 by IEEE Press, New York as a "Classic Reissue". .
Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are the materials that are noticeably attracted to them. Relatively few materials are ferromagnetic, and usually are pure forms, alloys, or compounds of iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
, nickel, and certain rare-earth metals. Beyond its chemical composition, a material's ferromagnetic properties (or lack thereof) is affected by its crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns t ...
. Ferromagnetism is very important in industry and modern technology and is the basis for many electrical and electromechanical devices such as electromagnets, electric motor
An electric motor is an electrical machine that converts electrical energy into mechanical energy. Most electric motors operate through the interaction between the motor's magnetic field and electric current in a wire winding to generate forc ...
s, generators, transformers, and magnetic storage such as tape recorders, and hard disks, and nondestructive testing of ferrous materials.
Ferromagnetic materials can be divided into magnetically "soft" materials like annealed iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, which can be magnetized but do not tend to stay magnetized, and magnetically "hard" materials, which do. Permanent magnets are made from "hard" ferromagnetic materials such as alnico and ferrimagnetic materials such as ferrite that are subjected to special processing in a strong magnetic field during manufacture to align their internal microcrystalline structure, making them very hard to demagnetize. To demagnetize a saturated magnet, a certain magnetic field must be applied, and this threshold depends on coercivity
Coercivity, also called the magnetic coercivity, coercive field or coercive force, is a measure of the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. Coercivity is usually measured in ...
of the respective material. "Hard" materials have high coercivity, whereas "soft" materials have low coercivity. The overall strength of a magnet is measured by its magnetic moment or, alternatively, the total magnetic flux it produces. The local strength of magnetism in a material is measured by its magnetization.
History and distinction from ferrimagnetism
Historically, the term ''ferromagnetism'' was used for any material that could exhibit spontaneous magnetization: a net magnetic moment in the absence of an external magnetic field; that is any material that could become a magnet. This general definition is still in common use. However, in a landmark paper in 1948, Louis Néel showed that there are two levels of magnetic alignment that result in this behavior. One is ferromagnetism in the strict sense, where all the magnetic moments are aligned. The other is '' ferrimagnetism'', where some magnetic moments point in the opposite direction but have a smaller contribution, so there is still a spontaneous magnetization. In the special case where the opposing moments balance completely, the alignment is known as '' antiferromagnetism''. Therefore antiferromagnets do not have a spontaneous magnetization.Ferromagnetic materials
Ferromagnetism is an unusual property that occurs in only a few substances. The common ones are thetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
s iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, nickel, cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
and their alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductilit ...
s, and alloys of rare-earth metals. It is a property not just of the chemical make-up of a material, but of its crystalline structure and microstructure. Their ferromagnetism results from having many unpaired electrons in their d-block in the case of iron and its relatives, or the f-block in the case of the rare-earth metals, a result of Hund's rule of maximum multiplicity. There are ferromagnetic metal alloys whose constituents are not themselves ferromagnetic, called Heusler alloys, named after Fritz Heusler
Carl Ludwig David Friedrich Heusler (1 February 1866, Dillenburg – 25 October 1947) was a German mining engineer and chemist. He discovered a special group of intermetallics now known as Heusler phases, which are ferromagnetic though the co ...
. Conversely, there are non-magnetic alloys, such as types of stainless steel, composed almost exclusively of ferromagnetic metals.
Amorphous (non-crystalline) ferromagnetic metallic alloys can be made by very rapid quenching
In materials science, quenching is the rapid cooling of a workpiece in water, oil, polymer, air, or other fluids to obtain certain material properties. A type of heat treating, quenching prevents undesired low-temperature processes, such as ...
(cooling) of a liquid alloy. These have the advantage that their properties are nearly isotropic (not aligned along a crystal axis); this results in low coercivity
Coercivity, also called the magnetic coercivity, coercive field or coercive force, is a measure of the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. Coercivity is usually measured in ...
, low hysteresis loss, high permeability, and high electrical resistivity. One such typical material is a transition metal- metalloid alloy, made from about 80% transition metal (usually Fe, Co, or Ni) and a metalloid component ( B, C, Si, P, or Al) that lowers the melting point.
A relatively new class of exceptionally strong ferromagnetic materials are the rare-earth magnets. They contain lanthanide elements that are known for their ability to carry large magnetic moments in well-localized f-orbitals.
The table lists a selection of ferromagnetic and ferrimagnetic compounds, along with the temperature above which they cease to exhibit spontaneous magnetization (see Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cu ...
).
Unusual materials
Most ferromagnetic materials are metals, since the conducting electrons are often responsible for mediating the ferromagnetic interactions. It is therefore a challenge to develop ferromagnetic insulators, especially multiferroic materials, which are both ferromagnetic and ferroelectric. A number of actinide compounds are ferromagnets at room temperature or exhibit ferromagnetism upon cooling. Pu P is a paramagnet with cubic symmetry at room temperature, but which undergoes a structural transition into a tetragonal state with ferromagnetic order when cooled below its ''T''C = 125 K. In its ferromagnetic state, PuP's easy axis is in the ⟨100⟩ direction. In NpFe2 the easy axis is ⟨111⟩. Above , NpFe2 is also paramagnetic and cubic. Cooling below the Curie temperature produces a rhombohedral distortion wherein the rhombohedral angle changes from 60° (cubic phase) to 60.53°. An alternate description of this distortion is to consider the length ''c'' along the unique trigonal axis (after the distortion has begun) and ''a'' as the distance in the plane perpendicular to ''c''. In the cubic phase this reduces to . Below theCurie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cu ...
:
which is the largest strain in any actinide compound. NpNi2 undergoes a similar lattice distortion below , with a strain of (43 ± 5) × 10−4. NpCo2 is a ferrimagnet below 15 K.
In 2009, a team of MIT physicists demonstrated that a lithium gas cooled to less than one kelvin can exhibit ferromagnetism. The team cooled fermionic lithium-6 to less than (150 billionths of one kelvin) using infrared laser cooling
Laser cooling includes a number of techniques in which atoms, molecules, and small mechanical systems are cooled, often approaching temperatures near absolute zero. Laser cooling techniques rely on the fact that when an object (usually an atom) ...
. This demonstration is the first time that ferromagnetism has been demonstrated in a gas.
In 2018, a team of University of Minnesota
The University of Minnesota, formally the University of Minnesota, Twin Cities, (UMN Twin Cities, the U of M, or Minnesota) is a public university, public Land-grant university, land-grant research university in the Minneapolis–Saint Paul, Tw ...
physicists demonstrated that body-centered tetragonal ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemic ...
exhibits ferromagnetism at room temperature.
Electrically induced ferromagnetism
Recent research has shown evidence that ferromagnetism can be induced in some materials by an electric current or voltage. Antiferromagnetic LaMnO3 and SrCoO have been switched to ferromagnetic by a current. In July 2020 scientists reported inducing ferromagnetism in the abundant diamagnetic material iron pyrite ("fool's gold") by an applied voltage. In these experiments the ferromagnetism was limited to a thin surface layer.Explanation
The Bohr–Van Leeuwen theorem, discovered in the 1910s, showed that classical physics theories are unable to account for any form of material magnetism, including ferromagnetism; the explanation rather depends on the quantum mechanical description ofatom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas ...
s. Each of an atom's electrons has a magnetic moment according to its spin state, as described by quantum mechanics. The Pauli exclusion principle, also a consequence of quantum mechanics, restricts the occupancy of electrons' spin states in atomic orbitals, generally causing the magnetic moments from an atom's electrons to largely or completely cancel. An atom will have a ''net'' magnetic moment when that cancellation is incomplete.
Origin of atomic magnetism
One of the fundamental properties of anelectron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
(besides that it carries charge) is that it has a magnetic dipole moment, i.e., it behaves like a tiny magnet, producing a magnetic field. This dipole moment comes from the more fundamental property of the electron that it has quantum mechanical spin. Due to its quantum nature, the spin of the electron can be in one of only two states; with the magnetic field either pointing "up" or "down" (for any choice of up and down). The spin of the electrons in atoms is the main source of ferromagnetism, although there is also a contribution from the orbital
Orbital may refer to:
Sciences Chemistry and physics
* Atomic orbital
* Molecular orbital
* Hybrid orbital Astronomy and space flight
* Orbit
** Earth orbit
Medicine and physiology
* Orbit (anatomy), also known as the ''orbital bone''
* Orbito ...
angular momentum
In physics, angular momentum (rarely, moment of momentum or rotational momentum) is the rotational analog of linear momentum. It is an important physical quantity because it is a conserved quantity—the total angular momentum of a closed sy ...
of the electron about the nucleus. When these magnetic dipoles in a piece of matter are aligned, (point in the same direction) their individually tiny magnetic fields add together to create a much larger macroscopic field.
However, materials made of atoms with filled electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's Atomic nucleus, nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by t ...
s have a total dipole moment of zero: because the electrons all exist in pairs with opposite spin, every electron's magnetic moment is cancelled by the opposite moment of the second electron in the pair. Only atoms with partially filled shells (i.e., unpaired spins) can have a net magnetic moment, so ferromagnetism occurs only in materials with partially filled shells. Because of Hund's rules
In atomic physics, Hund's rules refers to a set of rules that German physicist Friedrich Hund formulated around 1927, which are used to determine the term symbol that corresponds to the ground state of a multi- electron atom. The first rule is ...
, the first few electrons in a shell tend to have the same spin, thereby increasing the total dipole moment.
These unpaired dipoles (often called simply "spins", even though they also generally include orbital angular momentum) tend to align in parallel to an external magnetic field leading to a macroscopic effect called paramagnetism. In ferromagnetism, however, the magnetic interaction between neighboring atoms' magnetic dipoles is strong enough that they align with ''each other'' regardless of any applied field, resulting in the spontaneous magnetization of so-called domains. This results in the large observed magnetic permeability of ferromagnetics, and the ability of "hard" magnetic materials to form permanent magnets.
Exchange interaction
When two nearby atoms have unpaired electrons, whether the electron spins are parallel or antiparallel affects whether the electrons can share the same orbit as a result of the quantum mechanical effect called the exchange interaction. This in turn affects the electron location and the Coulomb (electrostatic) interaction and thus the energy difference between these states. The exchange interaction is related to the Pauli exclusion principle, which says that two electrons with the same spin cannot also be in the same spatial state (orbital). This is a consequence of the spin–statistics theorem and that electrons are fermions. Therefore, under certain conditions, when the orbitals of the unpaired outervalence electron
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair f ...
s from adjacent atoms overlap, the distributions of their electric charge in space are farther apart when the electrons have parallel spins than when they have opposite spins. This reduces the electrostatic energy of the electrons when their spins are parallel compared to their energy when the spins are antiparallel, so the parallel-spin state is more stable. This difference in energy is called the exchange energy. In simple terms, the outer electrons of adjacent atoms, which repel each other, can move further apart by aligning their spins in parallel, so the spins of these electrons tend to line up.
This energy difference can be orders of magnitude larger than the energy differences associated with the magnetic dipole–dipole interaction due to dipole orientation, which tends to align the dipoles antiparallel. In certain doped semiconductor oxides RKKY interaction RKKY stands for ''Ruderman–Kittel–Kasuya–Yosida.'' It refers to a coupling mechanism of nuclear magnetic moments or localized inner d- or f-shell electron spins in a metal by means of an interaction through the conduction electrons.
The RKK ...
s have been shown to bring about periodic longer-range magnetic interactions, a phenomenon of significance in the study of spintronic materials.
The materials in which the exchange interaction is much stronger than the competing dipole–dipole interaction are frequently called ''magnetic materials''. For instance, in iron (Fe) the exchange force is about 1000 times stronger than the dipole interaction. Therefore, below the Curie temperature virtually all of the dipoles in a ferromagnetic material will be aligned. In addition to ferromagnetism, the exchange interaction is also responsible for the other types of spontaneous ordering of atomic magnetic moments occurring in magnetic solids, antiferromagnetism and ferrimagnetism.
There are different exchange interaction mechanisms which create the magnetism in different ferromagnetic, ferrimagnetic, and antiferromagnetic substances. These mechanisms include direct exchange
Direct may refer to:
Mathematics
* Directed set, in order theory
* Direct limit of (pre), sheaves
* Direct sum of modules, a construction in abstract algebra which combines several vector spaces
Computing
* Direct access (disambiguation), ...
, RKKY exchange, double exchange, and superexchange.
Magnetic anisotropy
Although the exchange interaction keeps spins aligned, it does not align them in a particular direction. Without magnetic anisotropy, the spins in a magnet randomly change direction in response to thermal fluctuations and the magnet is superparamagnetic. There are several kinds of magnetic anisotropy, the most common of which is magnetocrystalline anisotropy. This is a dependence of the energy on the direction of magnetization relative to the crystallographic lattice. Another common source of anisotropy,inverse magnetostriction The inverse magnetostrictive effect, magnetoelastic effect or Villari effect, after its discoverer Emilio Villari, is the change of the magnetic susceptibility of a material when subjected to a mechanical stress.
Explanation
The magnetostriction ...
, is induced by internal strains. Single-domain magnets also can have a ''shape anisotropy'' due to the magnetostatic effects of the particle shape. As the temperature of a magnet increases, the anisotropy tends to decrease, and there is often a blocking temperature
In radiometric dating, closure temperature or blocking temperature refers to the temperature of a system, such as a mineral, at the time given by its radiometric date. In physical terms, the closure temperature is the temperature at which a syste ...
at which a transition to superparamagnetism occurs.
Magnetic domains
 The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called '' magnetic domains''
(also known as ''Weiss domains''). Within each domain, the spins are aligned, but (if the bulk material is in its lowest energy configuration; i.e. ''unmagnetized''), the spins of separate domains point in different directions and their magnetic fields cancel out, so the object has no net large scale magnetic field.
Ferromagnetic materials spontaneously divide into magnetic domains because the '' exchange interaction'' is a short-range force, so over long distances of many atoms the tendency of the magnetic dipoles to reduce their energy by orienting in opposite directions wins out. If all the dipoles in a piece of ferromagnetic material are aligned parallel, it creates a large magnetic field extending into the space around it. This contains a lot of magnetostatic energy. The material can reduce this energy by splitting into many domains pointing in different directions, so the magnetic field is confined to small local fields in the material, reducing the volume of the field. The domains are separated by thin domain walls a number of molecules thick, in which the direction of magnetization of the dipoles rotates smoothly from one domain's direction to the other.
The above would seem to suggest that every piece of ferromagnetic material should have a strong magnetic field, since all the spins are aligned, yet iron and other ferromagnets are often found in an "unmagnetized" state. The reason for this is that a bulk piece of ferromagnetic material is divided into tiny regions called '' magnetic domains''
(also known as ''Weiss domains''). Within each domain, the spins are aligned, but (if the bulk material is in its lowest energy configuration; i.e. ''unmagnetized''), the spins of separate domains point in different directions and their magnetic fields cancel out, so the object has no net large scale magnetic field.
Ferromagnetic materials spontaneously divide into magnetic domains because the '' exchange interaction'' is a short-range force, so over long distances of many atoms the tendency of the magnetic dipoles to reduce their energy by orienting in opposite directions wins out. If all the dipoles in a piece of ferromagnetic material are aligned parallel, it creates a large magnetic field extending into the space around it. This contains a lot of magnetostatic energy. The material can reduce this energy by splitting into many domains pointing in different directions, so the magnetic field is confined to small local fields in the material, reducing the volume of the field. The domains are separated by thin domain walls a number of molecules thick, in which the direction of magnetization of the dipoles rotates smoothly from one domain's direction to the other.
Magnetized materials
 Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the Barkhausen effect: as the magnetizing field is changed, the magnetization changes in thousands of tiny discontinuous jumps as the domain walls suddenly "snap" past defects.
This magnetization as a function of the external field is described by a hysteresis curve. Although this state of aligned domains found in a piece of magnetized ferromagnetic material is not a minimal-energy configuration, it is
Thus, a piece of iron in its lowest energy state ("unmagnetized") generally has little or no net magnetic field. However, the magnetic domains in a material are not fixed in place; they are simply regions where the spins of the electrons have aligned spontaneously due to their magnetic fields, and thus can be altered by an external magnetic field. If a strong enough external magnetic field is applied to the material, the domain walls will move by the process of the spins of the electrons in atoms near the wall in one domain turning under the influence of the external field to face in the same direction as the electrons in the other domain, thus reorienting the domains so more of the dipoles are aligned with the external field. The domains will remain aligned when the external field is removed, creating a magnetic field of their own extending into the space around the material, thus creating a "permanent" magnet. The domains do not go back to their original minimum energy configuration when the field is removed because the domain walls tend to become 'pinned' or 'snagged' on defects in the crystal lattice, preserving their parallel orientation. This is shown by the Barkhausen effect: as the magnetizing field is changed, the magnetization changes in thousands of tiny discontinuous jumps as the domain walls suddenly "snap" past defects.
This magnetization as a function of the external field is described by a hysteresis curve. Although this state of aligned domains found in a piece of magnetized ferromagnetic material is not a minimal-energy configuration, it is metastable
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
, and can persist for long periods, as shown by samples of magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With ...
from the sea floor which have maintained their magnetization for millions of years.
Heating and then cooling ( annealing) a magnetized material, subjecting it to vibration by hammering it, or applying a rapidly oscillating magnetic field from a degaussing coil tends to release the domain walls from their pinned state, and the domain boundaries tend to move back to a lower energy configuration with less external magnetic field, thus '' demagnetizing'' the material.
Commercial magnets are made of "hard" ferromagnetic or ferrimagnetic materials with very large magnetic anisotropy such as alnico and ferrites, which have a very strong tendency for the magnetization to be pointed along one axis of the crystal, the "easy axis". During manufacture the materials are subjected to various metallurgical processes in a powerful magnetic field, which aligns the crystal grains so their "easy" axes of magnetization all point in the same direction. Thus the magnetization, and the resulting magnetic field, is "built in" to the crystal structure of the material, making it very difficult to demagnetize.
Curie temperature
As the temperature increases, thermal motion, orentropy
Entropy is a scientific concept, as well as a measurable physical property, that is most commonly associated with a state of disorder, randomness, or uncertainty. The term and the concept are used in diverse fields, from classical thermodyna ...
, competes with the ferromagnetic tendency for dipoles to align. When the temperature rises beyond a certain point, called the Curie temperature
In physics and materials science, the Curie temperature (''T''C), or Curie point, is the temperature above which certain materials lose their permanent magnetic properties, which can (in most cases) be replaced by induced magnetism. The Cu ...
, there is a second-order phase transition and the system can no longer maintain a spontaneous magnetization, so its ability to be magnetized or attracted to a magnet disappears, although it still responds paramagnetically to an external field. Below that temperature, there is a spontaneous symmetry breaking and magnetic moments become aligned with their neighbors. The Curie temperature itself is a critical point, where the magnetic susceptibility is theoretically infinite and, although there is no net magnetization, domain-like spin correlations fluctuate at all length scales.
The study of ferromagnetic phase transitions, especially via the simplified Ising spin model, had an important impact on the development of statistical physics. There, it was first clearly shown that mean field theory approaches failed to predict the correct behavior at the critical point (which was found to fall under a ''universality class'' that includes many other systems, such as liquid-gas transitions), and had to be replaced by renormalization group theory.
See also
* * * * * *References
External links
*Electromagnetism
– ch. 11, from an online textbook * Detailed nonmathematical description of ferromagnetic materials with illustrations
Magnetism: Models and Mechanisms
in E. Pavarini, E. Koch, and U. Schollwöck: Emergent Phenomena in Correlated Matter, Jülich 2013, {{Authority control Quantum phases Magnetic hysteresis Physical phenomena Ferromagnetism