FeCl3 on:
[Wikipedia]
[Google]
[Amazon]
Iron(III) chloride is the inorganic compound with the formula . Also called ferric chloride, it is a common compound of iron in the +3
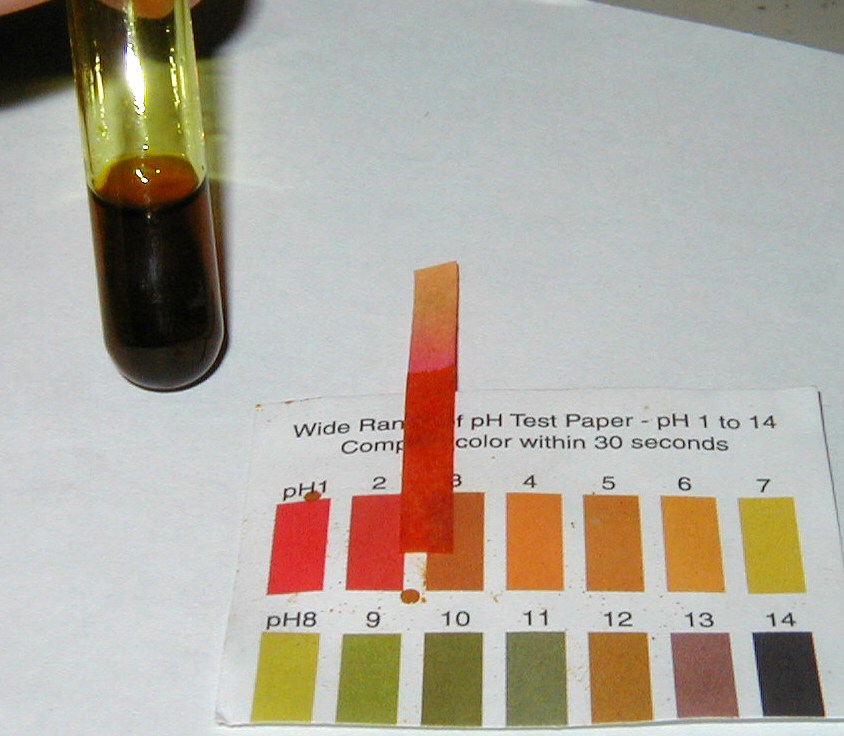 When dissolved in water, iron(III) chloride give a strongly acidic solution.
When heated with
When dissolved in water, iron(III) chloride give a strongly acidic solution.
When heated with
oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
. The anhydrous
A substance is anhydrous if it contains no water. Many processes in chemistry can be impeded by the presence of water; therefore, it is important that water-free reagents and techniques are used. In practice, however, it is very difficult to achie ...
compound is a crystalline solid with a melting point of 307.6 °C. The colour depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light
Transmittance of the surface of a material is its effectiveness in transmitting radiant energy. It is the fraction of incident electromagnetic power that is transmitted through a sample, in contrast to the transmission coefficient, which is th ...
they appear purple-red.
Structure and properties
Anhydrous
Anhydrous iron(III) chloride has the structure, with octahedral Fe(III) centres interconnected by two-coordinate chlorideligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s.
Iron(III) chloride has a relatively low melting point and boils at around 315 °C. The vapor consists of the dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
(like aluminium chloride) which increasingly dissociates into the monomeric (with D3h point group
In geometry, a point group is a mathematical group of symmetry operations (isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every p ...
molecular symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain m ...
) at higher temperature, in competition with its reversible decomposition to give iron(II) chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as ...
and chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
gas.
Hydrates
In addition to the anhydrous material, ferric chloride forms fourhydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
s. All forms of iron(III) chloride feature two or more chlorides as ligands, and three hydrates feature .
*''dihydrate'': has the structural formula ''trans''-.
* has the structural formula ''cis''-.
* has the structural formula ''cis''-.
*''hexahydrate'': has the structural formula ''trans''-.
Aqueous solution
Aqueous solutions of ferric chloride are characteristically yellow, in contrast to the pale pink solutions of . According to spectroscopic measurements, the main species in aqueous solutions of ferric chloride are the octahedral complex (stereochemistry unspecified) and the tetrahedral .Preparation
Anhydrous iron(III) chloride may be prepared by treating iron with chlorine: : Solutions of iron(III) chloride are produced industrially both from iron and from ore, in a closed-loop process. #Dissolvingiron ore
Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the fo ...
in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
#:
#Oxidation of iron(II) chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as ...
with chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
#:
#Oxidation of iron(II) chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as ...
with oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
and hydrochloric acid
#:
Heating hydrated iron(III) chloride does not yield anhydrous ferric chloride. Instead, the solid decomposes into hydrochloric acid and iron oxychloride
Iron oxychloride is the inorganic compound with the formula FeOCl. This purple solid adopts a layered structure, akin to that of cadmium chloride. The material slowly hydrolyses in moist air. The solid intercalates electron donors such as t ...
. Hydrated iron(III) chloride can be converted to the anhydrous form by treatment with thionyl chloride. Similarly, dehydration can be effected with trimethylsilyl chloride:
:
Reactions
iron(III) oxide
Iron(III) oxide or ferric oxide is the inorganic compound with the formula Fe2O3. It is one of the three main oxides of iron, the other two being iron(II) oxide (FeO), which is rare; and iron(II,III) oxide (Fe3O4), which also occurs naturally ...
at 350 °C, iron(III) chloride gives iron oxychloride
Iron oxychloride is the inorganic compound with the formula FeOCl. This purple solid adopts a layered structure, akin to that of cadmium chloride. The material slowly hydrolyses in moist air. The solid intercalates electron donors such as t ...
.
:
The anhydrous salt is a moderately strong Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
, forming adducts with Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s such as triphenylphosphine oxide; e.g., where Ph is phenyl. It also reacts with other chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
salts to give the yellow tetrahedral ion. Salts of in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
can be extracted into diethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
.
Redox reactions
Iron(III) chloride is a mildoxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ). In ot ...
, for example, it oxidizes copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gre ...
to copper(II) chloride
Copper(II) chloride is the chemical compound with the chemical formula CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate.
Both the anhydrous and the dihydrate forms occur naturally as the ver ...
.
:
In a comproportionation reaction, it reacts with iron to form iron(II) chloride:
:
A traditional synthesis of anhydrous ferrous chloride is the reduction of FeCl3 with chlorobenzene:
:
With carboxylate anions
Oxalates react rapidly with aqueous iron(III) chloride to give . Other carboxylate salts form complexes; e.g., citrate and tartrate.With alkali metal alkoxides
Alkali metalalkoxide
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, whe ...
s react to give the metal alkoxide complexes of varying complexity. The compounds can be dimeric or trimeric. In the solid phase a variety of multinuclear complexes have been described for the nominal stoichiometric reaction between and sodium ethoxide
Sodium ethoxide, also referred to as sodium ethylate, is the ionic, organic compound with the formula , or NaOEt (Et = ethane). It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. ...
:
:
With organometallic compounds
Iron(III) chloride inether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be c ...
solution oxidizes methyl lithium to give first light greenish yellow lithium tetrachloroferrate(III) solution and then, with further addition of methyl lithium, lithium tetrachloroferrate(II) :
:
:
The methyl radicals combine with themselves or react with other components to give mostly ethane and some methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
.
Uses
Industrial
Iron(III) chloride is used insewage treatment
Sewage treatment (or domestic wastewater treatment, municipal wastewater treatment) is a type of wastewater treatment which aims to remove contaminants from sewage to produce an effluent that is suitable for discharge to the surrounding envir ...
and drinking water production
Drinking is the act of ingesting water or other liquids into the body through the mouth, proboscis, or elsewhere. Humans drink by swallowing, completed by peristalsis in the esophagus. The physiological processes of drinking vary widely among ot ...
as a coagulant and flocculant. In this application, in slightly basic water reacts with the hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. I ...
ion () to form a floc of iron(III) hydroxide (), also formulated as FeO(OH) (ferrihydrite
Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydro ...
), that can remove suspended materials.
:
It is also used as a leaching
Leaching is the loss or extraction of certain materials from a carrier into a liquid (usually, but not always a solvent). and may refer to:
*Leaching (agriculture), the loss of water-soluble plant nutrients from the soil; or applying a small amoun ...
agent in chloride hydrometallurgy, for example in the production of Si from FeSi ( Silgrain process by Elkem
Elkem is a company that produces silicones, silicon, alloys for the foundry industry, carbon and microsilica, and other materials. Elkem was founded in 1904, has more than 7,000 employees and fields 30 production sites worldwide. Elkem has an oper ...
).
Another important application of iron(III) chloride is etching copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
in two-step redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction ...
reaction to copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gre ...
and then to copper(II) chloride
Copper(II) chloride is the chemical compound with the chemical formula CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate.
Both the anhydrous and the dihydrate forms occur naturally as the ver ...
in the production of printed circuit boards (PCB).
:
:
Iron(III) chloride is used as catalyst for the reaction of ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene i ...
with chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
, forming ethylene dichloride (1,2-dichloroethane
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl ...
), an important commodity chemical
Commodity chemicals (or bulk commodities or bulk chemicals) are a group of chemicals that are made on a very large scale to satisfy global markets. The average prices of commodity chemicals are regularly published in the chemical trade magazines an ...
, which is mainly used for the industrial production of vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
, the monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
for making PVC.
:
Laboratory use
In the laboratory iron(III) chloride is commonly employed as aLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
for catalysing reactions such as chlorination of aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping ...
s and Friedel–Crafts reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions ...
of aromatics. It is less powerful than aluminium chloride, but in some cases this mildness leads to higher yields, for example in the alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting ...
of benzene:
:
The ferric chloride test The ferric chloride test is used to determine the presence of phenols in a given sample or compound (for instance natural phenols in a plant extract). Enols, hydroxamic acids, oximes, and sulfinic acids give positive results as well. The bromine ...
is a traditional colorimetric
Colorimetry is "the science and technology used to quantify and describe physically the human color perception".
It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color ...
test for phenols, which uses a 1% iron(III) chloride solution that has been neutralized with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and alkali ...
until a slight precipitate of FeO(OH) is formed. The mixture is filtered before use. The organic substance is dissolved in water, methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
or ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ...
, then the neutralized iron(III) chloride solution is added—a transient or permanent coloration (usually purple, green or blue) indicates the presence of a phenol or enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene ( olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
.
This reaction is exploited in the Trinder spot test
The Trinder spot test is a diagnostic test used in medicine to determine exposure to salicylates, particularly to salicylic acid. The test employs the Trinder reagent (a.k.a. Trinder solution) which is mixed with a patient's urine. The colour ch ...
, which is used to indicate the presence of salicylates, particularly salicylic acid
Salicylic acid is an organic compound with the formula HOC6H4CO2H. A colorless, bitter-tasting solid, it is a precursor to and a metabolite of aspirin (acetylsalicylic acid). It is a plant hormone, and has been listed by the EPA Toxic Substance ...
, which contains a phenolic OH group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
.
This test can be used to detect the presence of gamma-hydroxybutyric acid
''gamma''-Hydroxybutyric acid (or γ-hydroxybutyric acid (GHB), also known as 4-hydroxybutanoic acid) is a naturally occurring neurotransmitter and a depressant drug. It is a precursor to GABA, glutamate, and glycine in certain brain areas. ...
and gamma-butyrolactone, which cause it to turn red-brown.
Other uses
* Used in anhydrous form as a drying reagent in certain reactions. * Used to detect the presence of phenol compounds inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
; e.g., examining purity of synthesized Aspirin
Aspirin, also known as acetylsalicylic acid (ASA), is a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and/or inflammation, and as an antithrombotic. Specific inflammatory conditions which aspirin is used to treat inc ...
.
* Used in water and wastewater treatment to precipitate phosphate as iron(III) phosphate.
* Used in wastewater treatment for odor control.
* Used by American coin collectors to identify the dates of Buffalo nickels that are so badly worn that the date is no longer visible.
* Used by bladesmiths and artisans in pattern welding
Pattern welding is the practice in sword and knife making of forming a blade of several metal pieces of differing composition that are forge welding, forge-welded together and twisted and manipulated to form a pattern. Often mistakenly called Dam ...
to etch the metal, giving it a contrasting effect, to view metal layering or imperfections.
* Used to etch the widmanstatten pattern in iron meteorites
A meteorite is a solid piece of debris from an object, such as a comet, asteroid, or meteoroid, that originates in outer space and survives its passage through the atmosphere to reach the surface of a planet or moon. When the original object en ...
.
* Necessary for the etching of photogravure plates for printing photographic and fine art images in intaglio and for etching rotogravure cylinders used in the printing industry.
* Used to make printed circuit board
A printed circuit board (PCB; also printed wiring board or PWB) is a medium used in Electrical engineering, electrical and electronic engineering to connect electronic components to one another in a controlled manner. It takes the form of a L ...
s (PCBs) by etching copper.
* Used to strip aluminum coating from mirrors.
* Used to etch intricate medical devices.
* Used in veterinary practice to treat overcropping of an animal's claws, particularly when the overcropping results in bleeding.
* Reacts with cyclopentadienylmagnesium bromide in one preparation of ferrocene
Ferrocene is an organometallic compound with the formula . The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, a ...
, a metal-sandwich complex.
* Sometimes used in a technique of Raku ware
is a type of Japanese pottery traditionally used in Japanese tea ceremonies, most often in the form of ''chawan'' tea bowls. It is traditionally characterised by being hand-shaped rather than thrown, fairly porous vessels, which result from low ...
firing, the iron coloring a pottery piece shades of pink, brown, and orange.
* Used to test the pitting and crevice corrosion resistance of stainless steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's corros ...
s and other alloy
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, ...
s.
* Used in conjunction with NaI
Nai or NAI may refer to:
Music
* ''Nai'' (album), an album by singer Anna Vissi
* Nai (pan flute), a wind instrument, also known as a pan flute (Romania and Moldova)
* "Nai" (song), a 2007 CD single by Irini Merkouri
Organizations
* National A ...
in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not clas ...
to mildly reduce organic azide
In chemistry, azide is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant applic ...
s to primary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s.
* Used in an animal thrombosis model.
* Used in an experimental energy storage systems.
* Historically it was used to make direct positive blueprints.
* A component of modified Carnoy's solution Carnoy's solution is a Fixation (histology), fixative composed of 60% ethanol, 30% chloroform and 10% glacial acetic acid, 1 gram of ferric chloride.
Carnoy's solution is also the name of a different fixation composed of ethanol and glacial acetic ...
used for surgical treatment of keratocystic odontogenic tumor
An odontogenic keratocyst is a rare and benign but locally aggressive developmental cyst. It most often affects the posterior mandible and most commonly presents in the third decade of life. Odontogenic keratocysts make up around 19% of jaw cysts. ...
(KOT).
* Used as an additive to sodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35.45 g ...
(NaCl) to produce clear crystals.
Safety
Iron(III) chloride is harmful, highly corrosive and acidic. The anhydrous material is a powerful dehydrating agent. Although reports of poisoning in humans are rare, ingestion of ferric chloride can result in serious morbidity and mortality. Inappropriate labeling and storage lead to accidental swallowing or misdiagnosis. Early diagnosis is important, especially in seriously poisoned patients.Natural occurrence
The natural counterpart of is the rare mineral molysite, usually related to volcanic and other-type fumaroles. is also produced as an atmospheric salt aerosol by reaction between iron-rich dust andhydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
from sea salt. This iron salt aerosol causes about 5% of naturally-occurring oxidization of methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
and is thought to have a range of cooling effects.
The atmosphere of the planet Venus
Venus is the second planet from the Sun. It is sometimes called Earth's "sister" or "twin" planet as it is almost as large and has a similar composition. As an interior planet to Earth, Venus (like Mercury) appears in Earth's sky never fa ...
is approximately 1% .
See also
* Verhoeff's stain * FerrosiliconNotes
References
Further reading
# # # # # # {{DEFAULTSORT:Iron(Iii) Chloride Chlorides Iron(III) compounds Metal halides Coordination complexes Deliquescent substances Dehydrating agents Acid catalysts