Fast Parallel Proteolysis (FASTpp) on:
[Wikipedia]
[Google]
[Amazon]
 Fast parallel proteolysis (FASTpp) is a method to determine the
Fast parallel proteolysis (FASTpp) is a method to determine the
 Fast parallel proteolysis (FASTpp) is a method to determine the
Fast parallel proteolysis (FASTpp) is a method to determine the thermostability
In materials science and molecular biology, thermostability is the ability of a substance to resist irreversible change in its chemical or physical structure, often by resisting decomposition or polymerization, at a high relative temperature ...
of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respon ...
s by measuring which fraction of protein resists rapid proteolytic digestion.
History and background
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called protease ...
is widely used in biochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology ...
and cell biology
Cell biology (also cellular biology or cytology) is a branch of biology that studies the structure, function, and behavior of cells. All living organisms are made of cells. A cell is the basic unit of life that is responsible for the living a ...
to probe protein structure
Protein structure is the molecular geometry, three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single ami ...
. In "limited trypsin proteolysis", low amounts of protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the form ...
digest both folded and unfolded protein but at largely different rates: unstructured proteins are cut more rapidly, while structured proteins are cut at a slower rate (sometimes by orders of magnitude). Recently, several other assays of protein stability based on proteolysis have been proposed, exploiting other proteases with high specificity for cleaving unfolded proteins. These include Pulse Proteolysis, Proteolytic Scanning Calorimetry and FASTpp.
How it works
FASTpp measures the quantity of protein that resists digestion under various conditions. To this end, a thermostable protease is used, which cleaves specifically at exposedhydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, ...
residues. The FASTpp assay combines the thermal unfolding, specificity of a thermostable protease for the unfolded fraction with the separation power of SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. ...
. Due to this combination, FASTpp can detect changes in the fraction folded over a large physico-chemical range of conditions including temperatures up to 85 °C, pH 6-9, presence or absence of the whole proteome
The proteome is the entire set of proteins that is, or can be, expressed by a genome, cell, tissue, or organism at a certain time. It is the set of expressed proteins in a given type of cell or organism, at a given time, under defined conditions. ...
. Applications range from biotechnology
Biotechnology is the integration of natural sciences and engineering sciences in order to achieve the application of organisms, cells, parts thereof and molecular analogues for products and services. The term ''biotechnology'' was first used b ...
to study of point mutations and ligand binding assay A ligand binding assay (LBA) is an assay, or an analytic procedure, which relies on the binding of ligand molecules to receptors, antibodies or other macromolecules. A detection method is used to determine the presence and extent of the ligand-rec ...
s.
Applications
FASTpp has been used to probe: * Lysate effect on protein stability * Thermal proteome stability * Coupled folding and binding * Ligand effects on fraction folded & stability * Effects of mutations on fraction folded & stability (e.g.point mutation
A point mutation is a genetic mutation where a single nucleotide base is changed, inserted or deleted from a DNA or RNA sequence of an organism's genome. Point mutations have a variety of effects on the downstream protein product—consequences ...
/ missense mutations)
* Kinetic protein stability
Technology
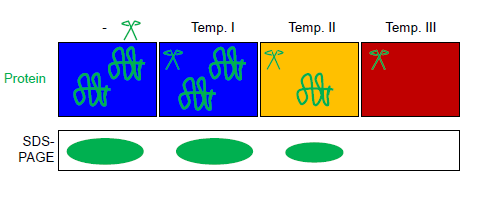
First, a cell lysate is generated by glass beads beating, pressure homogenisation or chemical or physical lysis methods that do not denature the protein(s) of interest. (Optionally for targeted analysis) a protein of interest is purified out of this lysate by affinity methods based on intrinsically disordered tags or other suitable purification strategies, often involving several orthogonal chromatographic steps. This (total or purified) protein solution is aliquoted into several tubes of a PCR strip. All aliquots are exposed in parallel in a thermal gradient PCR cycler to different maximal temperatures in presence of the thermostable protease thermolysin (see figure). Automated temperature control is achieved in a thermal gradient cycler (commonly used forPCR PCR or pcr may refer to:
Science
* Phosphocreatine, a phosphorylated creatine molecule
* Principal component regression, a statistical technique
Medicine
* Polymerase chain reaction
** COVID-19 testing, often performed using the polymerase chain r ...
s). Reaction products can be separated by SDS-PAGE
SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) is a discontinuous electrophoretic system developed by Ulrich K. Laemmli which is commonly used as a method to separate proteins with molecular masses between 5 and 250 kDa. ...
or western blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detec ...
. The protease thermolysin can be fully inactivated by EDTA
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid with the formula H2N(CH2CO2H)2sub>2. This white, water-soluble solid is widely used to bind to iron (Fe2+/Fe3+) and calcium ions (Ca2+), forming water-soluble complexes ev ...
. This feature of thermolysin makes FASTpp compatible with subsequent trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the d ...
digestion e.g. for mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
.
References
{{reflist, 2 Molecular biology techniques Proteomics Biophysics Biochemistry Structural biology