Eukaryotic Ribosome (80S) on:
[Wikipedia]
[Google]
[Amazon]

Ribosome
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to ...
s are a large and complex molecular machine that catalyzes the synthesis of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
s, referred to as translation
Translation is the communication of the Meaning (linguistic), meaning of a #Source and target languages, source-language text by means of an Dynamic and formal equivalence, equivalent #Source and target languages, target-language text. The ...
. The ribosome selects aminoacylated transfer RNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ac ...
s (tRNAs) based on the sequence of a protein-encoding messenger RNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of synthesizing a protein.
mRNA is created during the p ...
(mRNA) and covalently links the amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
s into a polypeptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A p ...
chain.
Ribosomes from all organisms share a highly conserved catalytic center. However, the ribosomes of eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s (animals, plants, fungi, and large number unicellular organisms all with a nucleus
Nucleus ( : nuclei) is a Latin word for the seed inside a fruit. It most often refers to:
*Atomic nucleus, the very dense central region of an atom
*Cell nucleus, a central organelle of a eukaryotic cell, containing most of the cell's DNA
Nucle ...
) are much larger than prokaryotic
A prokaryote () is a Unicellular organism, single-celled organism that lacks a cell nucleus, nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Greek language, Greek wikt:πρό#Ancient Greek, πρό (, 'before') a ...
(bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among ...
l and archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebac ...
l) ribosomes and subject to more complex regulation and biogenesis pathways.
Eukaryotic ribosomes are also known as 80S ribosomes, referring to their sedimentation coefficient
The sedimentation coefficient () of a particle characterizes its sedimentation during centrifugation. It is defined as the ratio of a particle's sedimentation velocity to the applied acceleration causing the sedimentation.
: s = \frac
The sedime ...
s in Svedberg units, because they sediment faster than the prokaryotic (70S
Ribosomes ( ) are macromolecular machines, found within all cells, that perform biological protein synthesis (mRNA translation). Ribosomes link amino acids together in the order specified by the codons of messenger RNA (mRNA) molecules to for ...
) ribosomes. Eukaryotic ribosomes have two unequal subunits, designated small subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex.
Large assemblies of proteins such as viruses often use a small number of ty ...
(40S) and large subunit
In structural biology, a protein subunit is a polypeptide chain or single protein molecule that assembles (or "''coassembles''") with others to form a protein complex.
Large assemblies of proteins such as viruses often use a small number of ty ...
(60S) according to their sedimentation coefficients. Both subunits contain dozens of ribosomal protein
A ribosomal protein (r-protein or rProtein) is any of the proteins that, in conjunction with rRNA, make up the ribosomal subunits involved in the cellular process of translation. ''E. coli'', other bacteria and Archaea have a 30S small subunit an ...
s arranged on a scaffold composed of ribosomal RNA
Ribosomal ribonucleic acid (rRNA) is a type of non-coding RNA which is the primary component of ribosomes, essential to all cells. rRNA is a ribozyme which carries out protein synthesis in ribosomes. Ribosomal RNA is transcribed from ribosomal ...
(rRNA). The small subunit monitors the complementarity between tRNA anticodon
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ac ...
and mRNA, while the large subunit catalyzes peptide bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein cha ...
formation.
Composition
Compared to their prokaryotic homologs, many of the eukaryotic ribosomal proteins are enlarged by insertions or extensions to the conserved core. Furthermore, several additional proteins are found in the small and large subunits of eukaryotic ribosomes, which do not have prokaryotic homologs. The 40S subunit contains a18S ribosomal RNA
18S ribosomal RNA (abbreviated 18S rRNA) is a part of the ribosomal RNA. The S in 18S represents Svedberg units. 18S rRNA is an SSU rRNA, a component of the eukaryotic ribosomal small subunit (40S). 18S rRNA is the structural RNA for the small c ...
(abbreviated 18S rRNA), which is homologous to the prokaryotic 16S rRNA 16S rRNA may refer to:
* 16S ribosomal RNA
16 S ribosomal RNA (or 16 S rRNA) is the RNA component of the 30S subunit of a prokaryotic ribosome ( SSU rRNA). It binds to the Shine-Dalgarno sequence and provides most of the SSU structure.
The g ...
. The 60S subunit contains a 28S rRNA that is homologous to the prokaryotic 23S ribosomal RNA
The 23S rRNA is a 2,904 nucleotide long (in '' E. coli'') component of the large subunit ( 50S) of the bacterial/archean ribosome and makes up the peptidyl transferase center (PTC). The 23S rRNA is divided into six secondary structural domains ...
. In addition, it contains a 5.8S rRNA that corresponds to the 5' end of the 23S rRNA, and a short 5S rRNA.
Both 18S and 28S have multiple insertions to the core rRNA fold of their prokaryotic counterparts, which are called expansion segments. For a detailed list of proteins, including archaeal and bacterial homologs please refer to the separate articles on the 40S
The eukaryotic small ribosomal subunit (40S) is the smaller subunit of the eukaryotic 80S ribosomes, with the other major component being the large ribosomal subunit (60S). The "40S" and "60S" names originate from the convention that ribosomal pa ...
and 60S subunits. Recent research suggests heterogeneity in the ribosomal composition, i.e., that the stoichiometry among core ribosomal proteins in wild-type yeast cells and embryonic stem cells depends both on the growth conditions and on the number of ribosomes bound per mRNA.
Structure determination
Initial structures of eukaryotic ribosomes were determined byelectron microscopy
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
.
First 3D structures were obtained at 30–40 Å resolution for yeast
and mammalian ribosomes.
Higher resolution structures of the yeast ribosome by cryo-electron microscopy
Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample sol ...
allowed the identification of protein and RNA structural elements.
More recently structures at sub-nanometer resolution were obtained for complexes of ribosomes and factors involved in translation.
After the determination of the first bacterial
and archaeal
ribosome structures at atomic resolution in the 1990s, it took another decade until in 2011, high resolution structures of eukaryotic ribosome were obtained by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
, mainly because of the difficulties in obtaining crystals of sufficient quality.
The complete structure of a eukaryotic 40S ribosomal structure in ''Tetrahymena thermophila
''Tetrahymena thermophila'' is a species of Ciliophora in the family Tetrahymenidae. It is a free living protozoa and occurs in fresh water.
There is little information on the ecology and natural history of this species, but it is the most wi ...
'' was published and described, as well as much about the 40S subunit's interaction with eIF1 during translation initiation. The eukaryotic 60S subunit structure was also determined from ''T. thermophila'' in complex with eIF6
Eukaryotic translation initiation factor 6 (EIF6), also known as Integrin beta 4 binding protein (ITGB4BP), is a human gene.
Hemidesmosomes are structures which link the basal lamina to the intermediate filament cytoskeleton. An important functio ...
. The complete structure of the eukaryotic 80S ribosome from the yeast ''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been o ...
'' was obtained by crystallography at 3.0 A resolution. These structures reveal the precise architecture of eukaryote-specific elements, their interaction with the universally conserved core, and all eukaryote-specific bridges between the two ribosomal subunits.
Atomic coordinates (PDB files) and structure factor
In condensed matter physics and crystallography, the static structure factor (or structure factor for short) is a mathematical description of how a material scatters incident radiation. The structure factor is a critical tool in the interpretation ...
s of the eukaryotic ribosome have been deposited in the Protein Data Bank (PDB) under the following accession codes:
Architecture
General features
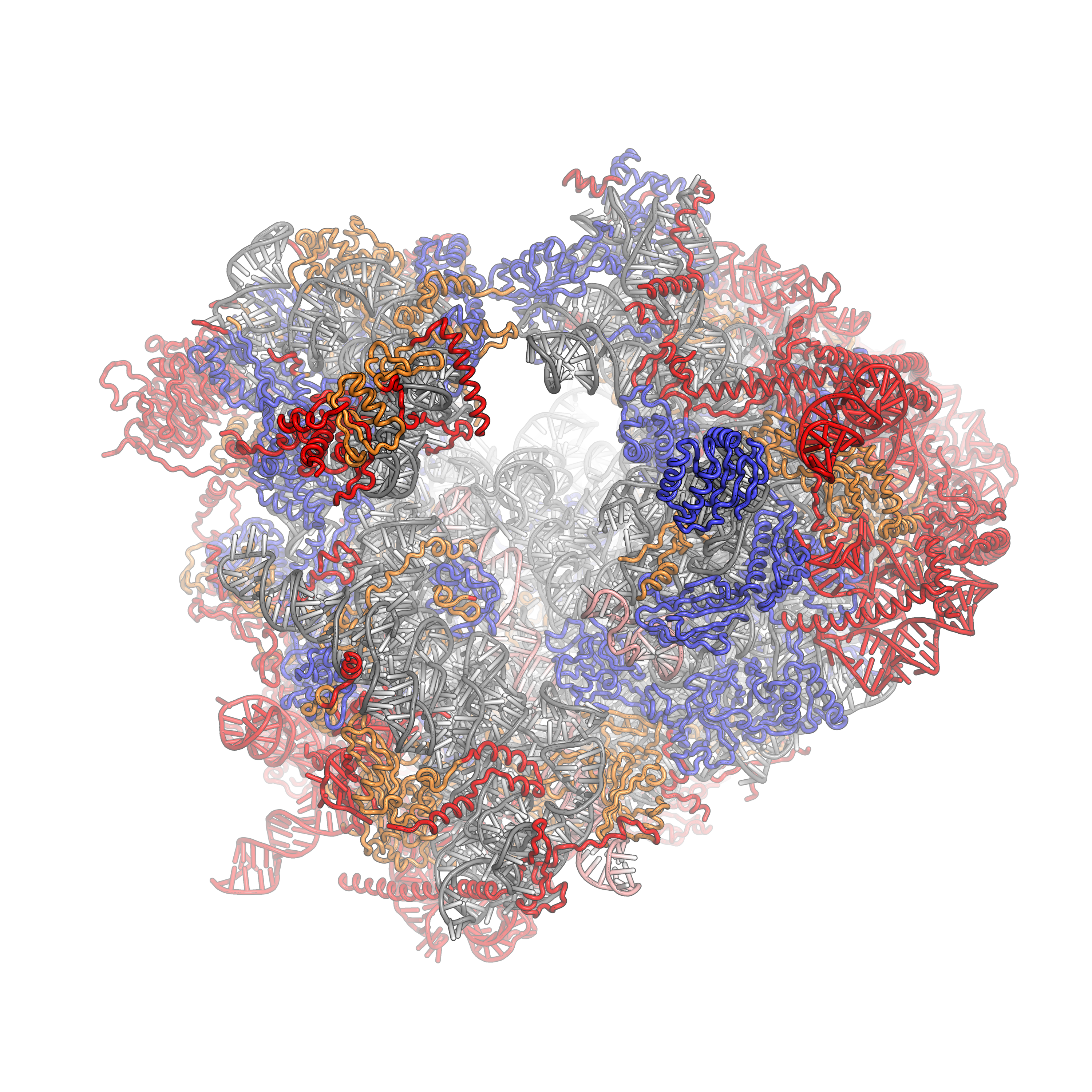
Some general architectural features of the ribosome are conserved across kingdoms: The structure of the small subunit can be sub-divided into two large segments, the head and the body. Characteristic features of the body include the left and right feet, the shoulder and the platform. The head features a pointed protrusion reminiscent of a bird's beak. In the characteristic "crown view" of the large subunit, structural landmarks include the central protuberance, the L1-stalk and the P-stalk. The majority of the eukaryote-specific RNA and protein elements are found on the solvent-exposed sides of the 40S and 60S subunits. The subunit interface, as well as important functional regions such as the peptidyl transferase center and the decoding site are mostly conserved, with some differences observed in the surrounding regions. In stark contrast to prokaryotic ribosomal proteins, which interact primarily with RNA, the eukaryote-specific protein segments engage in a multitude of protein-protein interactions. Long distance interactions are mediated by eukaryote-specifichelical
Helical may refer to:
* Helix, the mathematical concept for the shape
* Helical engine, a proposed spacecraft propulsion drive
* Helical spring, a coilspring
* Helical plc, a British property company, once a maker of steel bar stock
* Helicoil
A t ...
extensions of ribosomal proteins, and several eukaryotic ribosomal proteins jointly to form inter-protein beta-sheets.
The ribosomal RNA core is represented as a grey tube, expansion segments are shown in red. Universally conserved proteins are shown in blue. These proteins have homologs in eukaryotes, archaea and bacteria. Proteins Shared only between eukaryotes and archaea are shown in orange, and proteins specific to eukaryotes are shown in red.
Co-evolution of rRNA and proteins
The structure of the 40S subunit revealed that the eukaryote-specific proteins (rpS7, rpS10, rpS12 and RACK1), as well as numerous eukaryote-specific extensions of proteins, are located on the solvent-exposed side of the small subunit. Here, they participate in the stabilization of rRNA expansion segments. Moreover, the beak of the 40S subunit is remodeled, as rRNA has been replaced by proteins rpS10 and rpS12. As observed for the 40S subunit, all eukaryote-specific proteins of the 60S subunit (RPL6, RPL22, RPL27, RPL28, RPL29 and RPL36) and many extensions are located at the solvent-exposed side, forming an intricate network of interactions with eukaryotic-specific RNA expansion segments. RPL6, RPL27 and RPL29 mediate contacts between the ES sets ES7–ES39, ES31–ES20–ES26 and ES9–ES12, respectively and RPL28 stabilized expansion segment ES7A.Ubiquitin fusion proteins
In eukaryotes, the small subunit protein RPS27A (or eS31) and the large subunit protein RPL40 (or eL40) are processed polypeptides, which are translated asfusion protein
Fusion proteins or chimeric (kī-ˈmir-ik) proteins (literally, made of parts from different sources) are proteins created through the joining of two or more genes that originally coded for separate proteins. Translation of this ''fusion gene'' r ...
s carrying N-terminal ubiquitin
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Fo ...
domains. Both proteins are located next to important functional centers of the ribosome: the uncleaved ubiquitin domains of eS31) and eL40 would be positioned in the decoding site and near the translation factor binding site, respectively. These positions suggest that proteolytic cleavage is an essential step in the production of functional ribosomes. Indeed, mutations of the linker between the core of eS31 and the ubiquitin domain are lethal in yeast.
Active site
Comparisons between bacterial, archaeal and eukaryotic ribosome structures reveal a very high degree of conservation in the active site—aka thepeptidyl transferase
The peptidyl transferase is an aminoacyltransferase () as well as the primary enzymatic function of the ribosome, which forms peptide bonds between adjacent amino acids using tRNAs during the translation process of protein biosynthesis. The subst ...
center (PTC) -- region. None of the eukaryote-specific protein elements is close enough to directly participate in catalysis. However, RPL29 projects to within 18Å of the active site in ''T. thermophila'', and eukaryote-specific extensions interlink several proteins in the vicinity of the PTC of the 60S subunit, while the corresponding 50S proteins are singular entities.
Intersubunit bridges
Contacts across the two ribosomal subunits are known as intersubunit bridges. In the eukaryotic ribosome, additional contacts are made by 60S expansion segments and proteins. Specifically, the C-terminal extension of the 60S protein RPL19 interacts with ES6E of the 40S rRNA, and the C-terminal extension of the 60S protein RPL24 interacts with 40S rpS6 and rRNA helix h10. Moreover, the 60S expansion segments ES31 and ES41 interact with rpS3A(S1) and rpS8 of the 40S subunit, respectively, and the basic 25-amino-acid peptide RPL41 is positioned at the subunit interface in the 80S ribosome, interacting with rRNA elements of both subunits.Ribosomal proteins with roles in signaling
Two 40S ribosomal proteins (RACK1
Receptor for activated C kinase 1 (RACK1), also known as guanine nucleotide-binding protein subunit beta-2-like 1 (GNB2L1), is a 35 kDa protein that in humans is encoded by the RACK1 gene.
Function
RACK1 was originally isolated and identified a ...
and RPS6 (or eS6)) have been implicated in cellular signaling: RACK1, first described as the receptor of activated protein kinase C (PKC), is an integral component of the eukaryotic ribosome and is located at the back of the head. It may link signal-transduction pathways directly to the ribosome though it also has a role in multiple translational processes that appear unrelated (reviewed in ). Ribosomal protein eS6 is located at the right foot of the 40S subunit and is phosphorylated in response to mammalian target of rapamycin (mTOR) signaling.
Functional aspects
Translation initiation
Protein synthesis is primarily regulated at the stage of translation initiation. In eukaryotes, the canonical initiation pathway requires at least 12 proteininitiation factors Initiation factors are proteins that bind to the small subunit of the ribosome during the initiation of translation, a part of protein biosynthesis.
Initiation factors can interact with repressors to slow down or prevent translation. They have the ...
, some of which are themselves large complexes. The structures of the 40S:eIF1 and 60S:eIF6 complexes provide first detailed insights into the atomic interactions between the eukaryotic ribosome and regulatory factors. eIF1 is involved in start codon selection, and eIF6 sterically precludes the joining of subunits. However, structural information on the eukaryotic initiation factors and their interactions with the ribosome is limited and largely derived from homology models or low-resolution analyses. Elucidation of the interactions between the eukaryotic ribosome and initiation factors at an atomic level is essential for a mechanistic understanding of the regulatory processes, but represents a significant technical challenge, because of the inherent dynamics and flexibility of the initiation complexes. The first structure of the mammalian pre initiation complex was done by cryo-electron microscopy. Other structures of initiation complexes followed soon, driven by cryo-EM technical improvements.Hashem, Y., Des Georges, A., Dhote, V., Langlois, R., Liao, H. Y., Grassucci, R. A., ... & Frank, J. (2013). Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit. Nature. Those structures will help better understand the process of translation initiation in eukaryotes.
Regulatory roles of ribosomal proteins
Recent genetic evidence has been interpreted to suggest that individual proteins of the eukaryotic ribosome directly contribute to the regulation of translation. However, this interpretation is controversial and some researchers have proposed that genetic changes to ribosomal protein genes indirectly affect overall ribosome numbers or ribosome biogenesis processes.Protein translocation and targeting
To exert their functions in the cell newly synthesized proteins must be targeted to the appropriate location in the cell, which is achieved byprotein targeting
:''This article deals with protein targeting in eukaryotes unless specified otherwise.''
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the ce ...
and translocation systems. The growing polypeptide leaves the ribosome through a narrow tunnel in the large subunit. The region around the exit tunnel of the 60S subunit is very similar to the bacterial and archaeal 50S subunits. Additional elements are restricted to the second tier of proteins around the tunnel exit, possibly by conserved interactions with components of the translocation machinery. The targeting and translocation machinery is much more complex in eukaryotes.
Ribosomal diseases and cancer
Ribosomopathies are congenital human disorders resulting from defects in ribosomal protein or rRNA genes, or other genes whose products are implicated in ribosome biogenesis. Examples include X-linked Dyskeratosis congenita (X-DC),Diamond–Blackfan anemia
Diamond–Blackfan anemia (DBA) is a congenital erythroid aplasia that usually presents in infancy. DBA causes low red blood cell counts (anemia), without substantially affecting the other blood components (the platelets and the white blood cells) ...
, Treacher Collins syndrome (TCS) and Shwachman–Bodian–Diamond syndrome (SBDS). SBDS is caused by mutations in the SBDS protein that affects its ability to couple GTP hydrolysis by the GTPase EFL1 to the release of eIF6
Eukaryotic translation initiation factor 6 (EIF6), also known as Integrin beta 4 binding protein (ITGB4BP), is a human gene.
Hemidesmosomes are structures which link the basal lamina to the intermediate filament cytoskeleton. An important functio ...
from the 60S subunit.
Therapeutic opportunities
The ribosome is a prominent drug target forantibacterials
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
, which interfere with translation at different stages of the elongation cycle Most clinically relevant translation compounds are inhibitors of bacterial translation, but inhibitors of eukaryotic translation may also hold therapeutic potential for application in cancer or antifungal chemotherapy. Elongation inhibitors show antitumor activity 'in vivo' and 'in vitro'. One toxic inhibitor of eukaryotic translation elongation is the glutarimide
Glutarimide is the organic compound with the formula (CH2)3(CO)2NH. It is a white solid. The compound forms upon dehydration of the amide of glutaric acid.
Glutarimide is sometimes called 2,6-piperidinedione. It is the core of a variety of medi ...
antibiotic cycloheximide
Cycloheximide is a naturally occurring fungicide produced by the bacterium ''Streptomyces griseus''. Cycloheximide exerts its effects by interfering with the translocation step in protein synthesis (movement of two tRNA molecules and mRNA in rela ...
(CHX), which has been co-crystallized with the eukaryotic 60S subunit and binds in the ribosomal E site. The structural characterization of the eukaryotic ribosome may enable the use of structure-based methods for the design of novel antibacterials, wherein differences between the eukaryotic and bacterial ribosomes can be exploited to improve the selectivity of drugs and therefore reduce adverse effects
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complica ...
.
Formation mechanism
Eukaryote ribosomes are produced and assembled in thenucleolus
The nucleolus (, plural: nucleoli ) is the largest structure in the nucleus of eukaryotic cells. It is best known as the site of ribosome biogenesis, which is the synthesis of ribosomes. The nucleolus also participates in the formation of sig ...
. Ribosomal proteins enter the nucleolus and combine with the four rRNA strands to create the two ribosomal subunits (one small and one large) that will make up the completed ribosome. The ribosome units leave the nucleus through the nuclear pores
A nuclear pore is a part of a large complex of proteins, known as a nuclear pore complex that spans the nuclear envelope, which is the double membrane surrounding the eukaryotic cell nucleus. There are approximately 1,000 nuclear pore complexes ...
and unite once in the cytoplasm for the purpose of protein synthesis.
References
Notes
* * * {{Ribosome subunits Ribosomal RNA