erythrogenic toxin on:
[Wikipedia]
[Google]
[Amazon]
 Streptococcal pyrogenic exotoxins also known as erythrogenic toxins, are
Streptococcal pyrogenic exotoxins also known as erythrogenic toxins, are
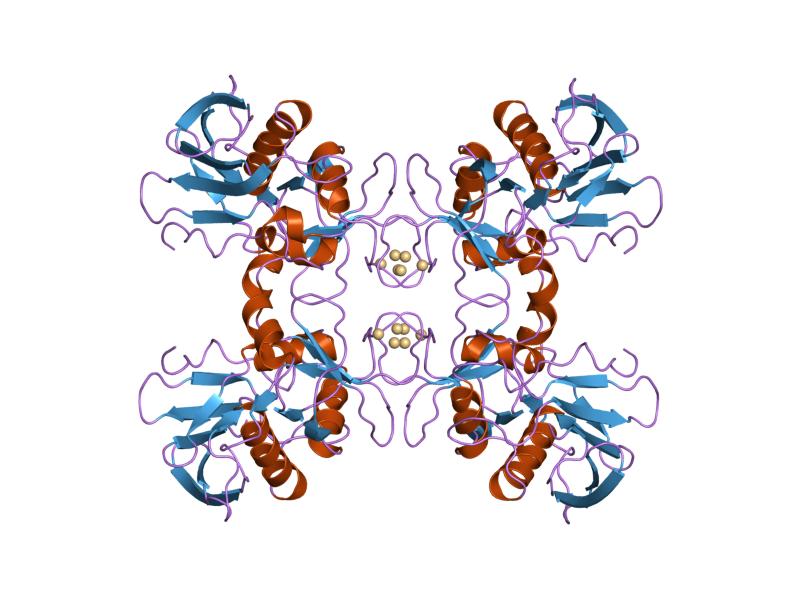
 SpeB is a 28 kDa protein with three major forms, mSpeB1, mSpeB2 and mSpeB3, which are categorized by variations the primary amino acid sequence. Three amino acids, C192, H340, and W357, are vital for enzymatic activity in all variants. The toxin contains a canonical papain-like domain, and mSpeB2 has an additional human
SpeB is a 28 kDa protein with three major forms, mSpeB1, mSpeB2 and mSpeB3, which are categorized by variations the primary amino acid sequence. Three amino acids, C192, H340, and W357, are vital for enzymatic activity in all variants. The toxin contains a canonical papain-like domain, and mSpeB2 has an additional human  All superantigenic streptococcal pyrogenic exotoxins contain two major conserved protein domains that are linked by an α-helix, which consist of an amino-terminal oligosoccharide/oligonucleotide binding fold and a carboxy-terminal β-grasp domain, as well as dodecapeptide binding region. SpeA also has a cystine loop, a low-affinity α-chain MHC II binding site, and the Vβ-TCR binding site. SpeC, SpeG, SpeH and SpeJ contains a Zn2+-dependent high β-chain MHC II binding site in addition to the low affinity site present in SpeA, and lacks the cystine loop. SpeH also has an additional α3-β8 loop that mediates the specificity of the toxin's Vβ-TCR binding site.
All superantigenic streptococcal pyrogenic exotoxins contain two major conserved protein domains that are linked by an α-helix, which consist of an amino-terminal oligosoccharide/oligonucleotide binding fold and a carboxy-terminal β-grasp domain, as well as dodecapeptide binding region. SpeA also has a cystine loop, a low-affinity α-chain MHC II binding site, and the Vβ-TCR binding site. SpeC, SpeG, SpeH and SpeJ contains a Zn2+-dependent high β-chain MHC II binding site in addition to the low affinity site present in SpeA, and lacks the cystine loop. SpeH also has an additional α3-β8 loop that mediates the specificity of the toxin's Vβ-TCR binding site.

Todar's Online Textbook of Bacteriology
{{Authority control Bacterial toxins Scarlet fever Proteins
 Streptococcal pyrogenic exotoxins also known as erythrogenic toxins, are
Streptococcal pyrogenic exotoxins also known as erythrogenic toxins, are exotoxin
An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, simi ...
s secreted by strains of the bacterial species ''Streptococcus pyogenes
''Streptococcus pyogenes'' is a species of Gram-positive, aerotolerant bacteria in the genus ''Streptococcus''. These bacteria are extracellular, and made up of non-motile and non-sporing cocci (round cells) that tend to link in chains. They are ...
''. SpeA and speC are superantigens
Superantigens (SAgs) are a class of antigens that result in excessive activation of the immune system. Specifically it causes non-specific activation of T-cells resulting in polyclonal T cell activation and massive cytokine release. SAgs are ...
, which induce inflammation by nonspecifically activating T cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell r ...
s and stimulating the production of inflammatory cytokines
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in autocrin ...
. SpeB, the most abundant streptococcal extracellular protein, is a cysteine protease
Cysteine proteases, also known as thiol proteases, are hydrolase enzymes that degrade proteins. These proteases share a common catalytic mechanism that involves a nucleophilic cysteine thiol in a catalytic triad or dyad.
Discovered by Gopal Chund ...
. Pyrogenic exotoxins are implicated as the causative agent of scarlet fever
Scarlet fever, also known as Scarlatina, is an infectious disease caused by ''Streptococcus pyogenes'' a Group A streptococcus (GAS). The infection is a type of Group A streptococcal infection (Group A strep). It most commonly affects childr ...
and streptococcal toxic shock syndrome
''Streptococcus'' is a genus of gram-positive ' (plural ) or spherical bacteria that belongs to the family Streptococcaceae, within the order Lactobacillales (lactic acid bacteria), in the phylum Bacillota. Cell division in streptococci occurs ...
. There is no consensus on the exact number of pyrogenic exotoxins. Serotypes A-C are the most extensively studied and recognized by all sources, but others note up to thirteen distinct types, categorizing speF through speM as additional superantigens.
Erythrogenic toxins are known to damage the plasma membranes of blood capillaries under the skin and produce a red skin rash (characteristic of scarlet fever). Past studies have shown that multiple variants of erythrogenic toxins may be produced, depending on the strain of ''S. pyogenes'' in question. Some strains may not produce a detectable toxin at all. Bacteriophage T12
Bacteriophage T12 is a bacteriophage that infects ''Streptococcus pyogenes'' bacteria. It is a proposed species of the family '' Siphoviridae'' in the order ''Caudovirales'' also known as ''tailed viruses''.NCBIBacteriophage T12 (species)/ref>
It ...
infection of ''S. pyogenes'' enables the production of speA, and increases virulence.
History
Discovery and nomenclature
SpeB was identified in 1919 as an ectoenzyme secreted by certain strains of streptococci. It was originally studied as two separate toxins, streptococcal pyrogenic exotoxin B and streptococcal cysteine proteinase, until it was shown that both proteins were encoded by the ''speB'' gene and that the attributed pyrogenic activities were due to contamination by SpeA and SpeC. ''Pyrogenic'', in ''streptococcal pyrogenic exotoxin'', means "causes fever." ''Erythrogenic'' refers to the typical red rash of scarlet fever. In older literature, these toxins are also referred to as ''scarlatina toxins'' or ''scarlet fever toxins'' due to their role as the causative agents of the disease. SpeB is known as ''streptococcal pyrogenic exotoxin B'', '' streptopain'' and ''streptococcal cysteine proteinase'' as a result of its original misidentification as two separate toxins, and is neither an exotoxin nor pyrogenic.Structure
Location of genes
The ''speB'' and ''speJ'' genes are located in the core bacterial chromosome of all strains of ''S. pyogenes.'' However, despite its presence and high levels of conservation in the nucleotide sequence, 25-40% of these strains do not express the SpeB toxin in significant amounts. In contrast, ''speA, speC'' and ''speH-M'' are encoded bybacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteri ...
s.
There is a lack of consensus over the location of the ''speG'' gene, which has been attributed to both the core chromosome and lysogenic phages.
Protein structure
 SpeB is a 28 kDa protein with three major forms, mSpeB1, mSpeB2 and mSpeB3, which are categorized by variations the primary amino acid sequence. Three amino acids, C192, H340, and W357, are vital for enzymatic activity in all variants. The toxin contains a canonical papain-like domain, and mSpeB2 has an additional human
SpeB is a 28 kDa protein with three major forms, mSpeB1, mSpeB2 and mSpeB3, which are categorized by variations the primary amino acid sequence. Three amino acids, C192, H340, and W357, are vital for enzymatic activity in all variants. The toxin contains a canonical papain-like domain, and mSpeB2 has an additional human integrin
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, ...
binding domain.
 All superantigenic streptococcal pyrogenic exotoxins contain two major conserved protein domains that are linked by an α-helix, which consist of an amino-terminal oligosoccharide/oligonucleotide binding fold and a carboxy-terminal β-grasp domain, as well as dodecapeptide binding region. SpeA also has a cystine loop, a low-affinity α-chain MHC II binding site, and the Vβ-TCR binding site. SpeC, SpeG, SpeH and SpeJ contains a Zn2+-dependent high β-chain MHC II binding site in addition to the low affinity site present in SpeA, and lacks the cystine loop. SpeH also has an additional α3-β8 loop that mediates the specificity of the toxin's Vβ-TCR binding site.
All superantigenic streptococcal pyrogenic exotoxins contain two major conserved protein domains that are linked by an α-helix, which consist of an amino-terminal oligosoccharide/oligonucleotide binding fold and a carboxy-terminal β-grasp domain, as well as dodecapeptide binding region. SpeA also has a cystine loop, a low-affinity α-chain MHC II binding site, and the Vβ-TCR binding site. SpeC, SpeG, SpeH and SpeJ contains a Zn2+-dependent high β-chain MHC II binding site in addition to the low affinity site present in SpeA, and lacks the cystine loop. SpeH also has an additional α3-β8 loop that mediates the specificity of the toxin's Vβ-TCR binding site.
Processing and regulation
The ''speB'' gene encodes for an amino acid sequence that becomes the 40 kDazymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active ...
, known as SpeBz, after cleavage of the signal sequence. SpeBz undergoes autocatalysis
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 199 ...
through at least eight intermediates to create the 28 kDa SpeBm. Finally cystine-192 and histidine-340 form a catalytic dyad. Each step is tightly regulated by multiple factors, allowing sophisticated temporal expression of the mature proteinase.
Mechanisms of action


SpeA and speC
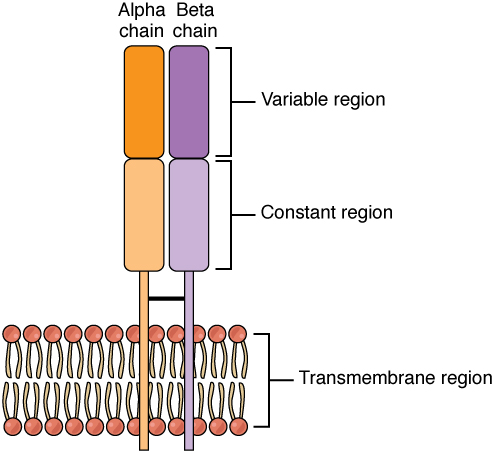
SpeA and SpeC bind toMHC Class II
MHC Class II molecules are a class of major histocompatibility complex (MHC) molecules normally found only on professional antigen-presenting cells such as dendritic cells, mononuclear phagocytes, some endothelial cells, thymic epithelial ce ...
molecules, are presented to T cells, and bind to the variable region of the beta chain of T-cell receptor
The T-cell receptor (TCR) is a protein complex found on the surface of T cells, or T lymphocytes, that is responsible for recognizing fragments of antigen as peptides bound to major histocompatibility complex (MHC) molecules. The binding b ...
s (TCRs). Once activated, the T cells release pro-inflammatory cytokines and chemokines. The interactions with TCRs are characterized by low affinities and fast dissociation, allowing the toxin to activate multiple T cells in succession. The lack of specificity allow the activation of up to 50% of the T cells in the body.
SpeB
SpeB cleaves degrades multiple proteins through hydrolysis, including cytokines, extracellular matrix proteins and immunoglobulin. It requires three amino acids before the cleavage site, known as P1, P2 and P3. Of these, SpeB has a preference for hydrophobic P2 and positively charged P1 residues, with greater importance of the P2 amino acid.Roles in virulence, pathogenesis and infection
SpeB
Streptococcal cysteine proteinase has roles in immune evasion and apoptosis, as well as potential influence on bacterial internalization. There is contradictory evidence regarding the effect of SpeB on virulence. Some studies have reported increased protease levels in strains that cause scarlet fever in comparison to those associated with streptococcal toxic shock syndrome, while others show decreased expression in more virulent strains. SpeB degrades immunoglobulins and cytokines, as well as through cleavage of C3b, inhibiting recruitment of phagocytic cells and the complement activation pathway. This results in decreased inflammation and neutrophil levels around the site of infection, preventing clearance and through phagocytosis and promoting the survival of ''S. pyogenes''. The toxin also inducesapoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
in host cells after GAS internalization. Evidence suggests that this may take place through extrinsic and intrinsic caspase pathways. The receptor-binding pathway and Fas-mediated apoptotic signaling pathway have been implicated in this process. The induction of apoptosis results in necrotizing fasciitis.
References
External links
*Todar's Online Textbook of Bacteriology
{{Authority control Bacterial toxins Scarlet fever Proteins