Dendrite (metal) on:
[Wikipedia]
[Google]
[Amazon]
A dendrite in
 The first computational model of dendritic solidification was published by Kobayashi,R. Kobayashi, Physica D., Vol. 63, 1993, pp. 410-423, https://doi.org/10.1016/0167-2789(93)90120-P who used a
The first computational model of dendritic solidification was published by Kobayashi,R. Kobayashi, Physica D., Vol. 63, 1993, pp. 410-423, https://doi.org/10.1016/0167-2789(93)90120-P who used a
metallurgy
Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
Metallurgy encompasses both the sc ...
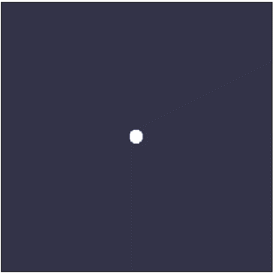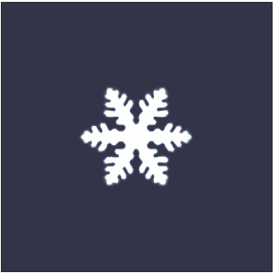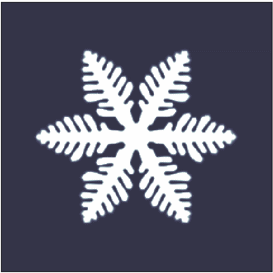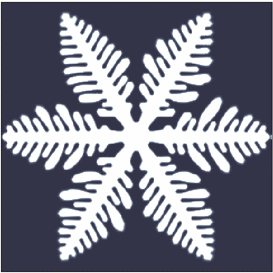
is a characteristic tree-like structure of crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s growing as molten metal
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
solidifies, the shape produced by faster growth along energetically favourable crystallographic
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics ( condensed matter physics). The w ...
directions. This dendritic growth has large consequences in regard to material properties.
Dendrites form in unary (one-component) systems as well as multi-component systems. The requirement is that the liquid (the molten material) be undercooled, aka supercooled, below the freezing point of the solid. Initially, a spherical solid nucleus grows in the undercooled melt. As the sphere grows, the spherical morphology becomes unstable and its shape becomes perturbed. The solid shape begins to express the preferred growth directions of the crystal. This growth direction may be due to anisotropy in the surface energy of the solid–liquid interface, or to the ease of attachment of atoms to the interface on different crystallographic planes, or both (for an example of the latter, see hopper crystal
A hopper crystal is a form of crystal, the shape of which resembles that of a pyramidal hopper container.
The edges of hopper crystals are fully developed, but the interior spaces are not filled in. This results in what appears to be a hollowed ...
). In metallic systems, interface attachment kinetics is usually negligible (for non-negligible cases, see dendrite (crystal)
A crystal dendrite is a crystal that develops with a typical multi-branching form. The name comes from the Greek word dendron (δενδρον) which means "tree", since the crystal's structure resembles that of a tree. These crystals can be synth ...
). In metallic systems, the solid then attempts to minimize the area of those surfaces with the highest surface energy. The dendrite thus exhibits a sharper and sharper tip as it grows. If the anisotropy is large enough, the dendrite may present a faceted morphology. The microstructural length scale is determined by the interplay or balance between the surface energy and the temperature gradient (which drives the heat/solute diffusion) in the liquid at the interface.
As solidification proceeds, an increasing number of atoms lose their kinetic energy, making the process exothermic. For a pure material, latent heat is released at the solid–liquid interface so that the temperature remains constant until the melt has completely solidified. The growth rate of the resultant crystalline substance will depend on how fast this latent heat can be conducted away. A dendrite growing in an undercooled melt can be approximated as a parabolic needle-like crystal that grows in a shape-preserving manner at constant velocity. Nucleation and growth determine the grain size in equiaxed solidification while the competition between adjacent dendrites decides the primary spacing in columnar growth. Generally, if the melt is cooled slowly, nucleation of new crystals will be less than at large undercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
. The dendritic growth will result in dendrites of a large size. Conversely, a rapid cooling cycle with a large undercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal ...
will increase the number of nuclei and thus reduce the size of the resulting dendrites (and often lead to small grains).
Smaller dendrites generally lead to higher ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
of the product. One application where dendritic growth and resulting material properties can be seen is the process of welding
Welding is a fabrication (metal), fabrication process that joins materials, usually metals or thermoplastics, by using high heat to melt the parts together and allowing them to cool, causing Fusion welding, fusion. Welding is distinct from lower ...
. The dendrites are also common in cast
Cast may refer to:
Music
* Cast (band), an English alternative rock band
* Cast (Mexican band), a progressive Mexican rock band
* The Cast, a Scottish musical duo: Mairi Campbell and Dave Francis
* ''Cast'', a 2012 album by Trespassers William
* ...
products, where they may become visible by etching of a polished specimen.
As dendrites develop further into the liquid metal, they get hotter because they continue to extract heat. If they get too hot, they will remelt. This remelting of the dendrites is called recalescence.
Dendrites usually form under non-equilibrium conditions.
An application of dendritic growth in directional solidification is gas turbine engine blades which are used at high temperatures and must handle high stresses along the major axes. At high temperatures, grain boundaries are weaker than grains. In order to minimize the effect on properties, grain boundaries are aligned parallel to the dendrites. The first alloy used in this application was a nickel-based alloy (MAR M-200) with 12.5% tungsten, which accumulated in the dendrites during solidification. This resulted in blades with high strength and creep resistance extending along the length of the casting, giving improved properties compared to the traditionally-cast equivalent.
Computational Modeling
 The first computational model of dendritic solidification was published by Kobayashi,R. Kobayashi, Physica D., Vol. 63, 1993, pp. 410-423, https://doi.org/10.1016/0167-2789(93)90120-P who used a
The first computational model of dendritic solidification was published by Kobayashi,R. Kobayashi, Physica D., Vol. 63, 1993, pp. 410-423, https://doi.org/10.1016/0167-2789(93)90120-P who used a phase-field model A phase-field model is a mathematical model for solving interfacial problems. It has mainly been applied to solidification dynamics, but it has also been applied to other situations such as viscous fingering, fracture mechanics, hydrogen embrittlem ...
to solve two coupled partial differential equations describing the evolution of the phase-field, (with in the liquid phase and in the solid phase), and the temperature field, , for a pure material in two dimensions:
:
which is an Allen-Cahn equation with an anisotropic gradient energy coefficient:
:
where is an average value of , is the angle between the interface normal and the x-axis, and and are constants representing the strength and mode of anisotropy, respectively.
The parameter describes the thermodynamic driving force for solidification, which Kobayashi defines for a supercooled melt as:
:
where is a constant between 0 and 1, is a positive constant, and is the dimensionless equilibrium temperature. The temperature has been non-dimensionalized such that the equilibrium temperature is and the initial temperature of the undercooled melt is .
The evolution equation for the temperature field is given by
:
and is simply the heat equation
In mathematics and physics, the heat equation is a certain partial differential equation. Solutions of the heat equation are sometimes known as caloric functions. The theory of the heat equation was first developed by Joseph Fourier in 1822 for t ...
with a source term due to the evolution of latent heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition.
Latent heat can be understo ...
upon solidification, where is a constant representing the latent heat normalized by the strength of the cooling.
When this system is numerically evolved, random noise representing thermal fluctuations is introduced to the interface via the term, where is the magnitude of the noise and is a random number distributed uniformly on