Cyclooctadecanonaene on:
[Wikipedia]
[Google]
[Amazon]
Cyclooctadecanonaene or 8nnulene is an
 Based on the enthalpy of hydrogenation, the overall
Based on the enthalpy of hydrogenation, the overall

organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
with chemical formula . It belongs to the class of highly conjugated compounds known as annulenes
Annulenes are monocyclic hydrocarbons that contain the maximum number of non-cumulated or conjugated double bonds (' mancude'). They have the general formula CnHn (when ''n'' is an even number) or C''n''H''n''+1 (when ''n'' is an odd number). The ...
and is aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
. The usual isomer that 8nnulene refers to is the most stable one, containing six interior hydrogens and twelve exterior ones, with the nine formal double bonds in the ''cis'',''trans'',''trans'',''cis'',''trans'',''trans'',''cis'',''trans'',''trans'' configuration. It is reported to be a red-brown crystalline solid.
Aromaticity
Notably, 8nnulene is the first annulene afterbenzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
( nnulene) to be fully aromatic
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to satur ...
: its π-system contains 4''n'' + 2 electrons (''n'' = 4), and it is large enough to comfortably accommodate six hydrogen atoms in its interior, allowing it to adopt a planar shape, thus satisfying Hückel's rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was fir ...
. The discovery of aromatic stabilization for 8nnulene is historically significant for confirming earlier theoretical predictions based on molecular orbital theory
In chemistry, molecular orbital theory (MO theory or MOT) is a method for describing the electronic structure of molecules using quantum mechanics. It was proposed early in the 20th century.
In molecular orbital theory, electrons in a molecule ...
, since simple versions of valence bond theory did not readily explain the 4''n'' + 2 rule.
The 1H NMR
Nuclear magnetic resonance (NMR) is a physical phenomenon in which nuclei in a strong constant magnetic field are perturbed by a weak oscillating magnetic field (in the near field) and respond by producing an electromagnetic signal with a ...
of this compound exhibits the hallmarks of a system with an aromatic ring current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons ...
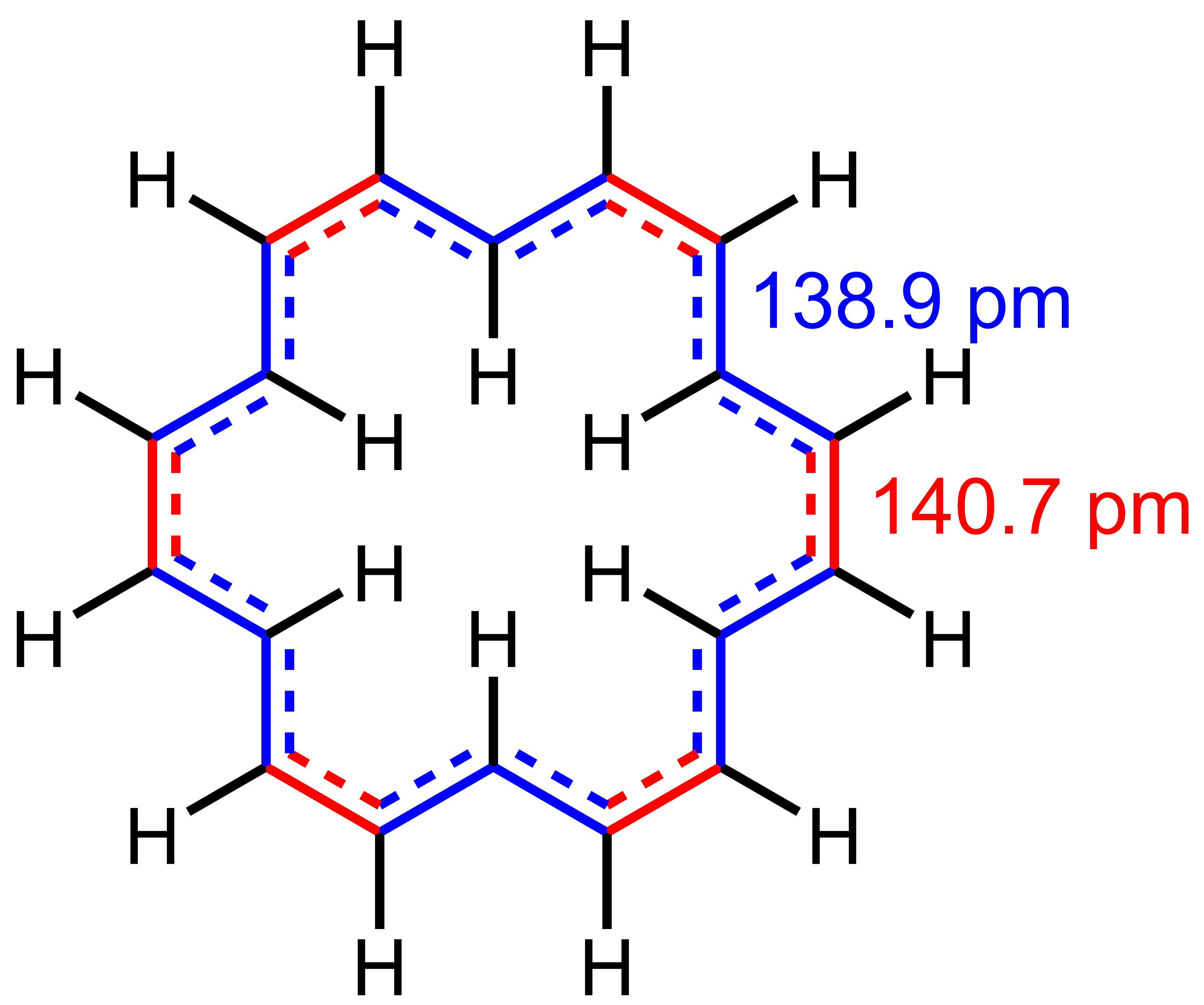
, with the 12H signal of the exterior hydrogens at 9.25 ppm, while the 6H signal of the interior hydrogens resonates at a remarkable −2.9 ppm in THF-''d8'' at −60 °C. On the other hand, a single signal at 5.45 ppm (the weighted average of the two individual signals) is observed at 120 °C. This is consistent with rapid exchange of the exterior and interior hydrogens at that temperature. The bond lengths in 8nnulene are in between those of single and double carbon–carbon bond, with two bond lengths observed crystallographically: 138.9 pm (concave edges) and 140.7 pm (convex edges). These bond lengths are indicative of significant delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
. The favorability of delocalization is, in turn, interpreted as evidence for aromaticity. For comparison, these values are close to the bond length of benzene (140 pm).
 Based on the enthalpy of hydrogenation, the overall
Based on the enthalpy of hydrogenation, the overall resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillatin ...
energy has been estimated to be 37 kcal/mol. This is about the same as that of benzene; however, this energy is spread out over 18 atoms instead of 6, so 8nnulene experiences a weaker stabilization than benzene. In terms of reactivity, it is somewhat more air- and light-stable than 4nnulene and 0nnulene, which are, respectively, weakly aromatic and nonaromatic due to transannular interactions. Nevertheless, it rapidly undergoes electrophilic addition
In organic chemistry, an electrophilic addition reaction is an addition reaction where a chemical compound containing a double or triple bond has a π bond broken, with the formation of two new σ bonds.March, Jerry; (1985). Advanced Organic Chem ...
s, much like other polyenes
In organic chemistry, polyenes are poly- unsaturated, organic compounds that contain at least three alternating double () and single () carbon–carbon bonds. These carbon–carbon double bonds interact in a process known as conjugation, resultin ...
, and attempts to effect Friedel-Crafts-type reactions on 8nnulene failed.
Despite the usual interpretation of 8nnulene as an 18-electron aromatic system, a 2014 theoretical study suggested that 8nnulene may be thought of as having only three completely delocalized π bonds
In chemistry, pi bonds (π bonds) are covalent bond, covalent chemical chemical bond, bonds, in each of which two lobes of an atomic orbital, orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap oc ...
associated with its aromaticity, while the other six π bonds represent conjugated three-center-two-electron ("3c-2e") π bonds on the periphery of the molecule.
Synthesis
The compound was first synthesised byFranz Sondheimer
Franz Sondheimer FRS (17 May 1926 – 11 February 1981) was a German-born British professor of chemistry. In 1960, he was awarded the Israel Prize for his contributions to science.
Biography
Franz Sondheimer was born in Stuttgart on 17 May 1926, ...
. The original synthesis started by the Eglinton reaction
The Glaser coupling is a type of coupling reaction. It is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) chloride or copper(I) bromide and an additional oxidant like oxygen. The base in its original scope is ammo ...
of the di-alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
1,5-hexadiyne with copper(II) acetate
Copper(II) acetate, also referred to as cupric acetate, is the chemical compound with the formula Cu(OAc)2 where AcO− is acetate (). The hydrated derivative, Cu2(OAc)4(H2O)2, which contains one molecule of water for each copper atom, is avail ...
in pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
to give the trimer, followed by deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
and isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeriz ...
with potassium ''tert''-butoxide in ''tert''-butanol and was concluded with hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, an ...
organic reduction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carr ...
with the Lindlar catalyst
A Lindlar catalyst is a heterogeneous catalyst that consists of palladium deposited on calcium carbonate or barium sulfate which is then poisoned with various forms of lead or sulfur. It is used for the hydrogenation of alkynes to alkenes (i.e. ...
.
:See also
* SuperbenzeneReferences