cyanation on:
[Wikipedia]
[Google]
[Amazon]
In organic synthesis, cyanation is the attachment or substitution of a
 A variety of mechanistically distinct pathways are known to cyanate arenes:
A variety of mechanistically distinct pathways are known to cyanate arenes:
 In addition, palladium-catalyzed cyanations of
In addition, palladium-catalyzed cyanations of  Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of
Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of 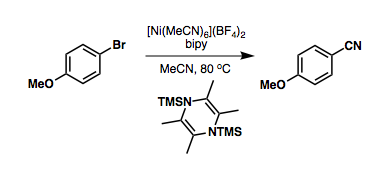 Sandmeyer cyanation is a means of converting
Sandmeyer cyanation is a means of converting


cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
group on various substrates. Such transformations are high-value because they generate C-C bond. Furthermore nitriles are versatile functional groups.
Cyanation to form sp3 nitriles
Typically, alkyl nitriles are formed ''via'' SN1 or SN2-type cyanation with alkyl electrophiles. Illustrative is the synthesis ofbenzyl cyanide
Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry.
Preparation
Benzyl cyanide can be produced via ...
by the reaction of benzyl chloride and sodium cyanide. In some cases cuprous cyanide
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper ...
is used instead of sodium cyanide.
Cyanation of ketones or aldehydes yields the corresponding cyanohydrins, which can be done directly with the cyanide ion (the cyanohydrin reaction
A cyanohydrin reaction is an organic chemical reaction in which an aldehyde or ketone reacts with a cyanide anion or a nitrile to form a cyanohydrin. This nucleophilic addition is a reversible reaction but with aliphatic carbonyl compounds eq ...
) or by using bisulfite, followed by displacement of sulfite:
A related reaction is hydrocyanation
In organic chemistry, hydrocyanation is a process for conversion of alkenes to nitriles. The reaction involves the addition of hydrogen cyanide and requires a catalyst. This conversion is conducted on an industrial scale for the production of ...
, which installs the elements of H-CN.
Cyanation of arenes
Cyanation of arenes offers access tobenzoic acid
Benzoic acid is a white (or colorless) solid organic compound with the formula , whose structure consists of a benzene ring () with a carboxyl () substituent. It is the simplest aromatic carboxylic acid. The name is derived from gum benzoin, ...
derivatives, as well as the utility of aryl nitriles themselves in as fine chemicals:
 A variety of mechanistically distinct pathways are known to cyanate arenes:
A variety of mechanistically distinct pathways are known to cyanate arenes:
With arene as two-electron electrophile
While the classical Rosenmund Von-Braun reaction utilizesstoichiometric
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equ ...
copper(I) cyanide
Copper(I) cyanide is an inorganic compound with the formula CuCN. This off-white solid occurs in two polymorphs; impure samples can be green due to the presence of Cu(II) impurities. The compound is useful as a catalyst, in electroplating copper ...
as a cyanation source, newer variants have been developed that are catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
in copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
:
 In addition, palladium-catalyzed cyanations of
In addition, palladium-catalyzed cyanations of aryl halide In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhi ...
s have been extensively explored. Generally, KCN or its less toxic surrogate Zn(CN)2 are used as nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
cyanide sources. To further diminish toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subs ...
concerns, potassium ferricyanide
Potassium ferricyanide is the chemical compound with the formula K3 e(CN)6 This bright red salt contains the octahedrally coordinated 3−.html" ;"title="e(CN)6sup>3−">e(CN)6sup>3− ion. It is soluble in water and its solution shows some g ...
has also been used as a cyanide source. Catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst. The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials s ...
s are believed to proceed through a standard Pd (0/II) pathway with reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
forging the key C-C bond. Deactivation of Pd(II) with excess cyanide is a common problem. Palladium catalysis conditions for aryl iodides, bromides, and even chlorides have been developed:
 Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of
Nickel-catalyzed cyanations avoid the use of precious metals, and can take advantage of benzyl cyanide
Benzyl cyanide (abbreviated BnCN) is an organic compound with the chemical formula C6H5CH2CN. This colorless oily aromatic liquid is an important precursor to numerous compounds in organic chemistry.
Preparation
Benzyl cyanide can be produced via ...
or acetonitrile as a cyanide source, ''via'' reductive C-C bond cleavage:
 Sandmeyer cyanation is a means of converting
Sandmeyer cyanation is a means of converting aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starti ...
derivatives to benzonitriles. The cyanation is generally postulated to be two-electron, while with radical mediators in absence of metals, the reaction is likely radical.
With arene as a two-electron nucleophile
Metalated arenes can be cyanated with electrophilic cyanide sources, includingcyanamides
Cyanamide is an organic compound with the formula C N2 H2. This white solid is widely used in agriculture and the production of pharmaceuticals and other organic compounds. It is also used as an alcohol-deterrent drug. The molecule features a ...
, cyanates
Cyanate is an anion with the structural formula , usually written . It also refers to any salt containing it, such as ammonium cyanate.
It is an isomer of the much less stable fulminate anion .William R. Martin and David W. Ball (2019): "Sma ...
, dimethylmalononitrile, or ethyl (ethoxymethylene)cyanoacetate. These methods can proceed with or without transition metal mediation:

With arene as a radical electrophile
Radical approaches to arene C-H cyanation are known. Photoredox mediators (metallic or organic) are most common:
References
{{Reflist Chemical reactions