Cupric Chloride on:
[Wikipedia]
[Google]
[Amazon]
Copper(II) chloride is the
Standard X-ray Diffraction Powder Patterns
National Bureau of Standards, Monograph 25, Section 18; page 33.
 Aqueous solution prepared from copper(II) chloride contain a range of copper(II) complexes depending on concentration, temperature, and the presence of additional chloride ions. These species include blue color of u(H2O)6sup>2+ and yellow or red color of the halide complexes of the formula uCl2+xsup>x−.
Aqueous solution prepared from copper(II) chloride contain a range of copper(II) complexes depending on concentration, temperature, and the presence of additional chloride ions. These species include blue color of u(H2O)6sup>2+ and yellow or red color of the halide complexes of the formula uCl2+xsup>x−.
 Partial hydrolysis gives
Partial hydrolysis gives
 This reaction is performed in a polar solvent such as
This reaction is performed in a polar solvent such as  Such compounds are intermediates in the synthesis of
Such compounds are intermediates in the synthesis of 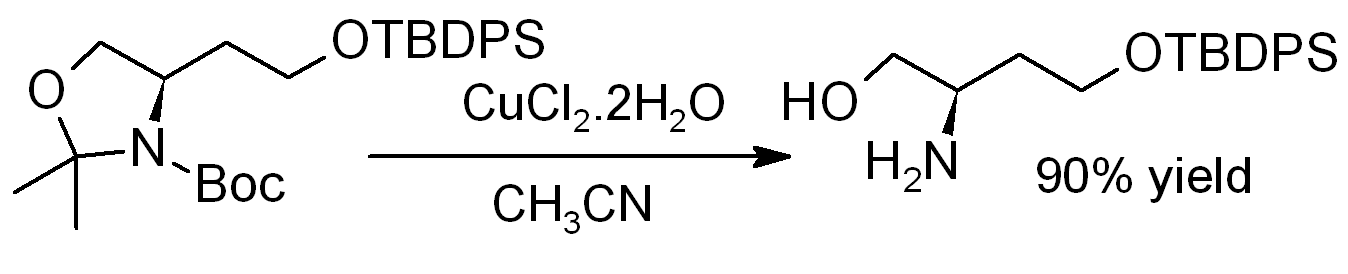 CuCl2 also catalyses the free radical addition of sulfonyl chlorides to
CuCl2 also catalyses the free radical addition of sulfonyl chlorides to
Copper Chloride
at '' The Periodic Table of Videos'' (University of Nottingham)
Copper (II) Chloride – Description and Pictures
{{Chlorides Copper(II) compounds Chlorides Metal halides Semiconductor materials Coordination complexes Pyrotechnic colorants
chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
CuCl2. The anhydrous form is yellowish brown but slowly absorbs moisture to form a blue-green dihydrate.
Both the anhydrous and the dihydrate forms occur naturally as the very rare minerals tolbachite and eriochalcite, respectively.Marlene C. Morris, Howard F. McMurdie, Eloise H. Evans, Boris Paretzkin, Harry S. Parker, and Nicolas C. Panagiotopoulos (1981) ''Copper chloride hydrate (eriochalcite)'', in Standard X-ray Diffraction Powder Patterns
National Bureau of Standards, Monograph 25, Section 18; page 33.
Structure
Anhydrous CuCl2 adopts a distorted cadmium iodide structure. In this motif, the copper centers are octahedral. Most copper(II) compounds exhibit distortions from idealized octahedral geometry due to the Jahn-Teller effect, which in this case describes the localization of one d-electron into amolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
that is strongly antibonding with respect to a pair of chloride ligands. In CuCl2·2H2O, the copper again adopts a highly distorted octahedral geometry, the Cu(II) centers being surrounded by two water ligands and four chloride ligands, which bridge
A bridge is a structure built to span a physical obstacle (such as a body of water, valley, road, or rail) without blocking the way underneath. It is constructed for the purpose of providing passage over the obstacle, which is usually somethi ...
asymmetrically to other Cu centers.
Copper(II) chloride is paramagnetic. Of historical interest, CuCl2·2H2O was used in the first electron paramagnetic resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...
measurements by Yevgeny Zavoisky
Yevgeny Konstantinovich Zavoisky (russian: Евгений Константинович Завойский; September 28, 1907 – October 9, 1976) was a Soviet physicist known for discovery of electron paramagnetic resonance in 1944. He likely obse ...
in 1944.
Properties and reactions
Hydrolysis
Copper(II) hydroxide
Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II) hydroxide are sold as "stabilized" copper(II) hydroxide, although they likely consist ...
precipitates upon treating copper(II) chloride solutions with base:
:CuCl2 + 2 NaOH → Cu(OH)2 + 2 NaCl
 Partial hydrolysis gives
Partial hydrolysis gives dicopper chloride trihydroxide
Dicopper chloride trihydroxide is the chemical compound with the formula Cu2(OH)3Cl. It is often referred to as tribasic copper chloride (TBCC), copper trihydroxyl chloride or copper hydroxychloride. It is a greenish crystalline solid encounte ...
, Cu2(OH)3Cl, a popular fungicide.
Redox
Copper(II) chloride is a mild oxidant. It decomposes tocopper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gre ...
and chlorine gas near 1000 °C:
:2 CuCl2 → 2 CuCl + Cl2
Copper(II) chloride (CuCl2) reacts with several metals to produce copper metal or copper(I) chloride (CuCl) with oxidation of the other metal. To convert copper(II) chloride to copper(I) chloride, it can be convenient to reduce an aqueous solution with sulfur dioxide
Sulfur dioxide (IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activ ...
as the reductant:
:2 CuCl2 + SO2 + 2 H2O → 2 CuCl + 2 HCl + H2SO4
Coordination complexes
CuCl2 reacts with HCl or otherchloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts ...
sources to form complex ions: the red CuCl3− (it is a dimer in reality, Cu2Cl62−, a couple of tetrahedrons that share an edge), and the green or yellow CuCl42−.
: +
: + 2
Some of these complexes can be crystallized from aqueous solution, and they adopt a wide variety of structures.
Copper(II) chloride also forms a variety of coordination complexes
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many m ...
with ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s such as ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
, pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a d ...
and triphenylphosphine oxide:
:CuCl2 + 2 C5H5N → uCl2(C5H5N)2(tetragonal)
:CuCl2 + 2 (C6H5)3PO → uCl2((C6H5)3PO)2(tetrahedral)
However "soft" ligands such as phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
s (e.g., triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists a ...
), iodide, and cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms.
In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of ...
as well as some tertiary amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
s induce reduction to give copper(I) complexes.
Preparation
Copper(II) chloride is prepared commercially by the action of chlorination of copper. Copper at red heat (300-400°C) combines directly with chlorine gas, giving (molten) copper (II) chloride. The reaction is very exothermic. : Cu(''s'') + Cl2(''g'') → CuCl2(''l'') It is also commercially practical to combinecopper(II) oxide
Copper(II) oxide or cupric oxide is an inorganic compound with the formula CuO. A black solid, it is one of the two stable oxides of copper, the other being Cu2O or copper(I) oxide (cuprous oxide). As a mineral, it is known as tenorite. It i ...
with an excess of ammonium chloride at similar temperatures, producing copper chloride, ammonia, and water:
: CuO + 2NH4Cl → CuCl2 + 2NH3 + H2O
Although copper metal itself cannot be oxidised by hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
, copper-containing bases such as the hydroxide, oxide, or copper(II) carbonate
Copper(II) carbonate or cupric carbonate is a chemical compound with formula . At ambient temperatures, it is an ionic solid (a salt) consisting of copper(II) cations and carbonate anions .
This compound is rarely encountered because it is diff ...
can react to form CuCl2 in an acid-base reaction.
Once prepared, a solution of CuCl2 may be purified by crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
. A standard method takes the solution mixed in hot dilute hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid
Acid strength is the tendency of an acid, symbol ...
, and causes the crystals to form by cooling in a Calcium chloride (CaCl2)-ice bath.S. H. Bertz, E. H. Fairchild, in ''Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation'', (R. M. Coates, S. E. Denmark, eds.), pp. 220-3, Wiley, New York, 1738.
There are indirect and rarely used means of using copper ions in solution to form copper(II) chloride. Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of aqueous sodium chloride with copper electrodes produces (among other things) a blue-green foam that can be collected and converted to the hydrate. While this is not usually done due to the emission of toxic chlorine gas, and the prevalence of the more general chloralkali process, the electrolysis will convert the copper metal to copper ions in solution forming the compound. Indeed, any solution of copper ions can be mixed with hydrochloric acid and made into a copper chloride by removing any other ions.
Natural occurrence
Copper(II) chloride occurs naturally as the very rare anhydrous mineral tolbachite and the dihydrate eriochalcite. Both are found near fumaroles and in some Cu mines. More common are mixed oxyhydroxide-chlorides likeatacamite
Atacamite is a copper halide mineral: a copper(II) chloride hydroxide with formula Cu2Cl(OH)3. It was first described for deposits in the Atacama Desert of Chile in 1801 by D. de Fallizen. The Atacama Desert is also the namesake of the mineral.
...
Cu2(OH)3Cl, arising among Cu ore beds oxidation zones in arid climate (also known from some altered slags).
Uses
In organic synthesis
Co-catalyst in Wacker process
A major industrial application for copper(II) chloride is as aco-catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
with palladium(II) chloride in the Wacker process
The Wacker process or the Hoechst-Wacker process (named after the chemical companies of the same name) refers to the oxidation of ethylene to acetaldehyde in the presence of palladium(II) chloride as the catalyst. This chemical reaction was one of ...
. In this process, ethene (ethylene) is converted to ethanal
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mo ...
(acetaldehyde) using water and air. During the reaction, PdCl2 is reduced to Pd, and the CuCl2 serves to re-oxidize this back to PdCl2. Air can then oxidize the resultant CuCl back to CuCl2, completing the cycle.
# C2H4 + PdCl2 + H2O → CH3CHO + Pd + 2 HCl
# Pd + 2 CuCl2 → 2 CuCl + PdCl2
# 4 CuCl + 4 HCl + O2 → 4 CuCl2 + 2 H2O
The overall process is:
:2 C2H4 + O2 → 2 CH3CHO
Other organic synthetic applications
Copper(II) chloride has some highly specialized applications in the synthesis of organic compounds. It affects chlorination of aromatic hydrocarbons—this is often performed in the presence of aluminium oxide. It is able to chlorinate the alpha position of carbonyl compounds: : This reaction is performed in a polar solvent such as
This reaction is performed in a polar solvent such as dimethylformamide
Dimethylformamide is an organic compound with the formula ( CH3)2NC(O)H. Commonly abbreviated as DMF (although this initialism is sometimes used for dimethylfuran, or dimethyl fumarate), this colourless liquid is miscible with water and the majo ...
(DMF), often in the presence of lithium chloride, which accelerates the reaction.
CuCl2, in the presence of oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, can also oxidize phenols. The major product can be directed to give either a quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds
or a coupled product from oxidative dimerization. The latter process provides a high-yield route to 1,1-binaphthol:
:uch as benzene or naphthalene
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab province. Uch may have been founded as Alexandria on the Indus, a town founded by Alexand ...
by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double ... Such compounds are intermediates in the synthesis of
Such compounds are intermediates in the synthesis of BINAP
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This chiral diphosphine ligand is widely used in asymmetric synthesis. It consists of a pair of 2-diphenylphosphinonaphthyl groups linked at the 1 and 1� ...
and its derivatives.
Copper(II) chloride dihydrate promotes the hydrolysis of acetonides, i.e., for deprotection to regenerate diols or aminoalcohols, as in this example (where TBDPS = ''tert''-butyldiphenylsilyl):
: CuCl2 also catalyses the free radical addition of sulfonyl chlorides to
CuCl2 also catalyses the free radical addition of sulfonyl chlorides to alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s; the alpha-chlorosulfone may then undergo elimination with base to give a vinyl sulfone product.
In inorganic synthesis
Catalyst in production of chlorine
Copper(II) chloride is used as a catalyst in a variety of processes that produce chlorine byoxychlorination In organic chemistry, oxychlorination is a process for making C-Cl bonds. In contrast with direct use of Cl2, oxychlorination uses hydrogen chloride in combination with oxygen.{{Ullmann, author=M. Rossberg , display-authors=et al., title=Chlorinated ...
. The Deacon process
The Deacon process, invented by Henry Deacon, is a process used during the manufacture of alkalis (the initial end product was sodium carbonate) by the Leblanc process. Hydrogen chloride gas was converted to chlorine gas, which was then used ...
takes place at about 400 to 450 °C in the presence of a copper chloride:
:4 HCl + O2 → 2 Cl2 + 2 H2O
Copper(II) chloride catalyzes the chlorination in the production of vinyl chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC ...
and dichloroethane.H.Wayne Richardson, "Copper Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim,
Copper(II) chloride is used in the Copper–chlorine cycle in which it splits steam into a copper oxygen compound and hydrogen chloride, and is later recovered in the cycle from the electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from n ...
of copper(I) chloride.
Niche uses
Copper(II) chloride is also used inpyrotechnics
Pyrotechnics is the science and craft of creating such things as fireworks, safety matches, oxygen candles, explosive bolts and other fasteners, parts of automotive airbags, as well as gas-pressure blasting in mining, quarrying, and demolition. ...
as a blue/green coloring agent. In a flame test, copper chlorides, like all copper compounds, emit green-blue.
In humidity indicator card
A humidity indicator card (HIC) is a card on which a moisture-sensitive chemical is impregnated such that it will change color when the indicated relative humidity is exceeded. This has usually been a blotting paper impregnated with cobalt(II) ch ...
s (HICs), cobalt-free brown to azure (copper(II) chloride base) HICs can be found on the market. In 1998, the European Community
The European Economic Community (EEC) was a regional organization created by the Treaty of Rome of 1957,Today the largely rewritten treaty continues in force as the ''Treaty on the functioning of the European Union'', as renamed by the Lisbo ...
(EC) classified items containing cobalt(II) chloride of 0.01 to 1% w/w as T (Toxic), with the corresponding R phrase
R-phrases (short for risk phrases) are defined in Annex III of European Union Directive 67/548/EEC: ''Nature of special risks attributed to dangerous substances and preparations''. The list was consolidated and republished in Directive 2001/59/EC, ...
of R49 (may cause cancer if inhaled). As a consequence, new cobalt-free humidity indicator cards have been developed that contain copper.
Safety
Copper(II) chloride can be toxic. Only concentrations below 5 ppm are allowed in drinking water by the US Environmental Protection Agency.References
Further reading
# # # ''The Merck Index'', 7th edition, Merck & Co, Rahway, New Jersey, USA, 1960. # D. Nicholls, ''Complexes and First-Row Transition Elements'', Macmillan Press, London, 1973. # A. F. Wells, Structural Inorganic Chemistry'', 5th ed., Oxford University Press, Oxford, UK, 1984. # J. March, ''Advanced Organic Chemistry'', 4th ed., p. 723, Wiley, New York, 1992. # ''Fieser & Fieser Reagents for Organic Synthesis'' Volume 5, p158, Wiley, New York, 1975. #External links
Copper Chloride
at '' The Periodic Table of Videos'' (University of Nottingham)
Copper (II) Chloride – Description and Pictures
{{Chlorides Copper(II) compounds Chlorides Metal halides Semiconductor materials Coordination complexes Pyrotechnic colorants