Control Blade on:
[Wikipedia]
[Google]
[Amazon]
 Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as
 Control rods are inserted into the core of a nuclear reactor and adjusted in order to
Control rods are inserted into the core of a nuclear reactor and adjusted in order to
 Chemical elements with usefully high neutron capture cross-sections include silver, indium, and cadmium. Other candidate elements include
Chemical elements with usefully high neutron capture cross-sections include silver, indium, and cadmium. Other candidate elements include
 Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as
Control rods are used in nuclear reactors to control the rate of fission of the nuclear fuel – uranium or plutonium. Their compositions include chemical elements such as boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
, cadmium, silver, hafnium, or indium, that are capable of absorbing many neutrons without themselves decaying. These elements have different neutron capture cross sections for neutrons of various energies. Boiling water reactors (BWR), pressurized water reactors (PWR), and heavy-water reactor
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water ( deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. T ...
s (HWR) operate with thermal neutrons, while breeder reactors operate with fast neutrons. Each reactor design can use different control rod materials based on the energy spectrum of its neutrons. Control rods have been used in nuclear aircraft engines like Project Pluto
Project Pluto was a United States government program to develop nuclear-powered ramjet engines for use in cruise missiles. Two experimental engines were tested at the Nevada Test Site (NTS) in 1961 and 1964 respectively.
On 1 January 1957, th ...
as a method of control.
Operating principle
 Control rods are inserted into the core of a nuclear reactor and adjusted in order to
Control rods are inserted into the core of a nuclear reactor and adjusted in order to control
Control may refer to:
Basic meanings Economics and business
* Control (management), an element of management
* Control, an element of management accounting
* Comptroller (or controller), a senior financial officer in an organization
* Controlling ...
the rate
Rate or rates may refer to:
Finance
* Rates (tax), a type of taxation system in the United Kingdom used to fund local government
* Exchange rate, rate at which one currency will be exchanged for another
Mathematics and science
* Rate (mathema ...
of the nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
and, thereby, the thermal power
A thermal power station is a type of power station in which heat energy
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary ...
output of the reactor, the rate of steam
Steam is a substance containing water in the gas phase, and sometimes also an aerosol of liquid water droplets, or air. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization ...
production, and the electrical power output of the power station.
The number of control rods inserted, and the distance to which they are inserted, strongly influence the ''reactivity'' of the reactor. When reactivity (as effective neutron multiplication factor
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
) is above 1, the rate of the nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
increases exponentially over time. When reactivity is below 1, the rate of the reaction decreases exponentially over time. When all control rods are fully inserted, they keep reactivity barely above 0, which quickly slows a running reactor to a stop and keeps it stopped (in shutdown
Shutdown or shut down may refer to:
* Government shutdowns in the United States
* Shutdown (computing)
* Shutdown (economics)
* Shutdown (nuclear reactor)
Arts and entertainment Music
* "Shut Down" (The Beach Boys song), 1963
* ''Shut Down Volu ...
). If all control rods are fully removed, reactivity is significantly above 1, and the reactor quickly runs hotter and hotter, until some other factor (such as temperature reactivity feedback) slows the reaction rate. Maintaining a constant power output requires keeping the long-term average neutron multiplication factor close to 1.
A new reactor is assembled with its control rods fully inserted. Control rods are partially removed from the core to allow the nuclear chain reaction
In nuclear physics, a nuclear chain reaction occurs when one single nuclear reaction causes an average of one or more subsequent nuclear reactions, thus leading to the possibility of a self-propagating series of these reactions. The specific nu ...
to start up and increase to the desired power level. Neutron flux can be measured, and is roughly proportional to reaction rate and power level. To increase power output, some control rods are pulled out a small distance for a while. To decrease power output, some control rods are pushed in a small distance for a while. Several other factors affect the reactivity; to compensate for them, an automatic control system adjusts the control rods small amounts in or out, as-needed in some reactors. Each control rod influences some part of the reactor more than others; calculated adjustments to fuel distribution can be made to maintain similar reaction rates and temperatures in different parts of the core.
Typical shutdown
Shutdown or shut down may refer to:
* Government shutdowns in the United States
* Shutdown (computing)
* Shutdown (economics)
* Shutdown (nuclear reactor)
Arts and entertainment Music
* "Shut Down" (The Beach Boys song), 1963
* ''Shut Down Volu ...
time for modern reactors such as the European Pressurized Reactor
The EPR is a Generation III reactor, third generation pressurised water reactor design. It has been designed and developed mainly by Framatome (part of Areva between 2001 and 2017) and Électricité de France (EDF) in France, and Siemens in Germ ...
or Advanced CANDU reactor is 2 seconds for 90% reduction, limited by decay heat
Decay heat is the heat released as a result of radioactive decay. This heat is produced as an effect of radiation on materials: the energy of the alpha, beta or gamma radiation is converted into the thermal movement of atoms.
Decay heat occurs na ...
.
Control rods are usually used in control rod assemblies (typically 20 rods for a commercial PWR assembly) and inserted into guide tubes within the fuel elements. Control rods often stand vertically within the core. In PWRs they are inserted from above, with the control rod drive mechanisms mounted on the reactor pressure vessel
A pressure vessel is a container designed to hold gases or liquids at a pressure substantially different from the ambient pressure.
Construction methods and materials may be chosen to suit the pressure application, and will depend on the size o ...
head. In BWRs, due to the necessity of a steam dryer above the core, this design requires insertion of the control rods from beneath.
Materials
 Chemical elements with usefully high neutron capture cross-sections include silver, indium, and cadmium. Other candidate elements include
Chemical elements with usefully high neutron capture cross-sections include silver, indium, and cadmium. Other candidate elements include boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
, cobalt, hafnium, samarium, europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, and lutetium. Alloys or compounds may also be used, such as high-boron steel
Boron steel refers to steel alloyed with a small amount of boron, usually less than 1%. The addition of boron to steel greatly increases the hardenability of the resulting alloy.
Description
Boron is added to steel as ferroboron (~12-24% B). As ...
, silver-indium-cadmium alloy, boron carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders,
as well as numerous industrial applications. With a Vickers hard ...
, zirconium diboride, titanium diboride, hafnium diboride
Hafnium diboride belongs to the class of ultra-high-temperature ceramics, a type of ceramic composed of hafnium and boron. It has a melting temperature of about 3250 °C. It is an unusual ceramic, having relatively high thermal and electrical c ...
, gadolinium nitrate, gadolinium titanate, dysprosium titanate
Dysprosium titanate ( Dy2 Ti2 O7) is an inorganic compound, a ceramic of the titanate family, with pyrochlore structure.
Dysprosium titanate, like holmium titanate and holmium stannate, is a spin ice material. In 2009, quasiparticles resembli ...
, and boron carbide–europium hexaboride composite.
The material choice is influenced by the neutron energy in the reactor, their resistance to neutron-induced swelling, and the required mechanical and lifespan properties. The rods may have the form of tubes filled with neutron-absorbing pellets or powder. The tubes can be made of stainless steel or other "neutron window" materials such as zirconium, chromium, silicon carbide, or cubic (cubic boron nitride).
The burnup of "burnable poison
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large neutron absorption cross-section. In such applications, absorbing neutrons is normally an undesirable eff ...
" isotopes also limits lifespan of a control rod. They may be reduced by using an element such as hafnium, a "non-burnable poison" which captures multiple neutrons before losing effectiveness, or by not using neutron absorbers for trimming. For example, in pebble bed reactors or in possible new type lithium-7-moderated and -cooled reactors that use fuel and absorber pebbles.
Some rare-earth elements are excellent neutron absorbers and are more common than silver (reserves of about 500,000t). For example, ytterbium (reserves about 1 M tons) and yttrium, 400 times more common, with middle capturing values, can be found and used together without separation inside minerals like xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as sili ...
(Yb) (Yb0.40Y0.27Lu0.12Er0.12Dy0.05Tm0.04Ho0.01)PO4, or keiviite (Yb) (Yb1.43Lu0.23Er0.17Tm0.08Y0.05Dy0.03Ho0.02)2Si2O7, lowering the cost. Xenon is also a strong neutron absorber as a gas, and can be used for controlling and (emergency) stopping helium-cooled reactors, but does not function in cases of pressure loss, or as a burning protection gas together with argon around the vessel part especially in case of core catching reactors or if filled with sodium or lithium. Fission-produced xenon can be used after waiting for caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
to precipitate, when practically no radioactivity is left. Cobalt-59 is also used as an absorber for winning of cobalt-60 for X-ray production. Control rods can also be constructed as thick turnable rods with a tungsten reflector and absorber side turned to stop by a spring in less than 1 second.
Silver-indium-cadmium alloys, generally 80% Ag, 15% In, and 5% Cd, are a common control rod material for pressurized water reactors. The somewhat different energy absorption regions of the materials make the alloy an excellent neutron absorber. It has good mechanical strength and can be easily fabricated. It must be encased in stainless steel to prevent corrosion in hot water. Although indium is less rare than silver, it is more expensive.
Boron is another common neutron absorber. Due to the different cross sections of 10B and 11B, materials containing boron enriched in 10B by isotopic separation are frequently used. The wide absorption spectrum of boron also makes it suitable as a neutron shield. The mechanical properties of boron in its elementary form are unsuitable, and therefore alloys or compounds have to be used instead. Common choices are high-boron steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
and boron carbide
Boron carbide (chemical formula approximately B4C) is an extremely hard boron–carbon ceramic, a covalent material used in tank armor, bulletproof vests, engine sabotage powders,
as well as numerous industrial applications. With a Vickers hard ...
. The latter is used as a control rod material in both PWRs and BWRs. 10B/11B separation is done commercially with gas centrifuges over BF3, but can also be done over BH3 from borane production or directly with an energy optimized melting centrifuge, using the heat of freshly separated boron for preheating.
Hafnium has excellent properties for reactors using water for both moderation and cooling. It has good mechanical strength, can be easily fabricated, and is resistant to corrosion in hot water. Hafnium can be alloyed with other elements, e.g. with tin and oxygen to increase tensile and creep strength, with iron, chromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hardne ...
, and niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has sim ...
for corrosion resistance, and with molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
for wear resistance, hardness, and machineability. Such alloys are designated as Hafaloy, Hafaloy-M, Hafaloy-N, and Hafaloy-NM. The high cost and low availability of hafnium limit its use in civilian reactors, although it is used in some US Navy reactors. Hafnium carbide can also be used as an insoluble material with a high melting point of 3890 °C and density higher than that of uranium dioxide for sinking, unmelted, through corium.
Dysprosium titanate
Dysprosium titanate ( Dy2 Ti2 O7) is an inorganic compound, a ceramic of the titanate family, with pyrochlore structure.
Dysprosium titanate, like holmium titanate and holmium stannate, is a spin ice material. In 2009, quasiparticles resembli ...
was undergoing evaluation for pressurized water control rods. Dysprosium titanate is a promising replacement for Ag-In-Cd alloys because it has a much higher melting point, does not tend to react with cladding materials, is easy to produce, does not produce radioactive waste, does not swell and does not outgas
Outgassing (sometimes called offgassing, particularly when in reference to indoor air quality) is the release of a gas that was dissolved, trapped, frozen, or absorbed in some material. Outgassing can include sublimation and evaporation (which ...
. It was developed in Russia and is recommended by some for VVER and RBMK reactors. A disadvantage is less titanium and oxide absorption, that other neutron absorbing elements do not react with the already high-melting point cladding materials and that just using the unseparated content with dysprosium inside of minerals like Keiviit Yb inside chromium, SiC or c11B15N tubes deliver superior price and absorption without swelling and outgassing.
Hafnium diboride
Hafnium diboride belongs to the class of ultra-high-temperature ceramics, a type of ceramic composed of hafnium and boron. It has a melting temperature of about 3250 °C. It is an unusual ceramic, having relatively high thermal and electrical c ...
is another such material. It can be used alone or in a sintered mixture of hafnium and boron carbide powders.
Many other compounds of rare-earth elements can be used, such as samarium with boron-like europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lanth ...
and samarium boride
Samarium is a chemical element with chemical symbol, symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. C ...
, which is already used in the colour industry. Less absorptive compounds of boron similar to titanium, but inexpensive, such as molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lea ...
as Mo2B5. Since they all swell with boron, in practice other compounds are better, such as carbides, etc., or compounds with two or more neutron-absorbing elements together. It is important that tungsten, and probably also other elements like tantalum, have much the same high capture qualities as hafnium, but with the opposite effect. This is not explainable by neutron reflection alone. An obvious explanation is resonance gamma rays increasing the fission and breeding ratio versus causing more capture of uranium, etc. over metastable conditions like for isotope 235mU, which has a half-life of about 26 min.
Additional means of reactivity regulation
Other means of controlling reactivity include (for PWR) a soluble neutron absorber ( boric acid) added to the reactor coolant, allowing the complete extraction of the control rods during stationary power operation, ensuring an even power and flux distribution over the entire core. Thischemical shim
In applications such as nuclear reactors, a neutron poison (also called a neutron absorber or a nuclear poison) is a substance with a large Neutron cross section, neutron absorption cross-section. In such applications, absorbing neutrons is norma ...
, along with the use of burnable neutron poisons within the fuel pellets, is used to assist regulation of the core's long term reactivity, while the control rods are used for rapid reactor power changes (e.g. shutdown and start up). Operators of BWRs use the coolant flow through the core to control reactivity by varying the speed of the reactor recirculation pumps (an increase in coolant flow through the core improves the removal of steam bubbles, thus increasing the density of the coolant/ moderator, increasing power).
Safety
In most reactor designs, as a safety measure, control rods are attached to the lifting machinery by electromagnets, rather than direct mechanical linkage. This means that in the event of power failure, or if manually invoked due to failure of the lifting machinery, the control rods fall automatically, under gravity, all the way into the pile to stop the reaction. A notable exception to this fail-safe mode of operation is the BWR, which requires hydraulic insertion in the event of an emergency shut-down, using water from a special tank under high pressure. Quickly shutting down a reactor in this way is called scramming.Criticality accident prevention
Mismanagement or control rod failure have often been blamed fornuclear accident
A nuclear and radiation accident is defined by the International Atomic Energy Agency (IAEA) as "an event that has led to significant consequences to people, the environment or the facility. Examples include lethal effects to individuals, lar ...
s, including the SL-1 explosion and the Chernobyl disaster
The Chernobyl disaster was a nuclear accident that occurred on 26 April 1986 at the No. 4 reactor in the Chernobyl Nuclear Power Plant, near the city of Pripyat in the north of the Ukrainian SSR in the Soviet Union. It is one of only two nuc ...
.
''Homogeneous'' neutron absorbers have often been used to manage criticality accidents which involve aqueous solutions of fissile metals. In several such accidents, either borax ( sodium borate) or a cadmium compound has been added to the system. The cadmium can be added as a metal to nitric acid solutions of fissile material; the corrosion of the cadmium in the acid will then generate cadmium nitrate
Nitrate is a polyatomic ion
A polyatomic ion, also known as a molecular ion, is a covalent bonded set of two or more atoms, or of a metal complex, that can be considered to behave as a single unit and that has a net charge that is not zer ...
''in situ''.
In carbon dioxide-cooled reactors such as the AGR, if the solid control rods fail to arrest the nuclear reaction, nitrogen gas can be injected into the primary coolant cycle. This is because nitrogen has a larger absorption cross-section for neutrons than carbon or oxygen; hence, the core then becomes less reactive.
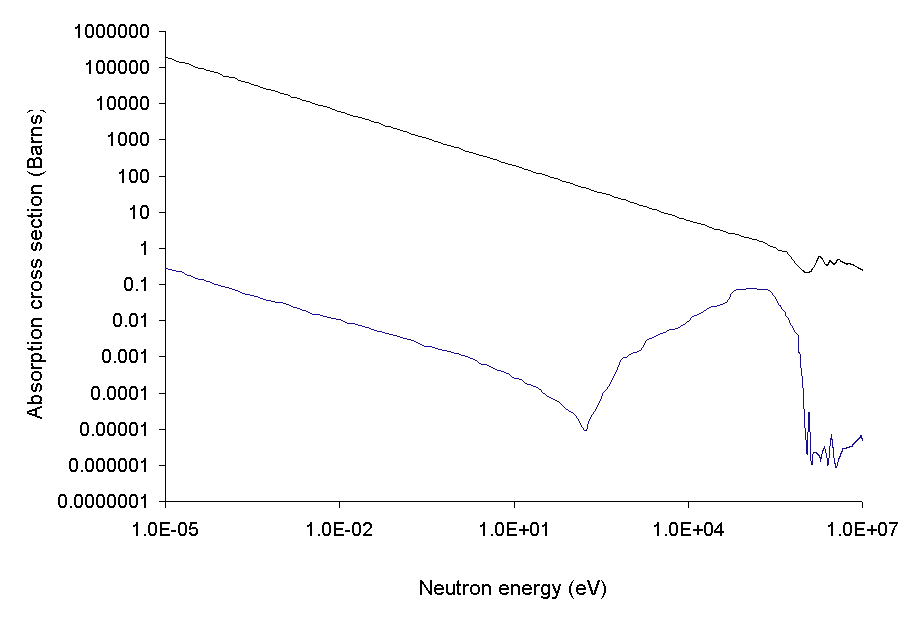
As the neutron energy increases, the neutron cross section of most isotopes decreases. The boron
Boron is a chemical element with the symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the ''boron group'' it has th ...
isotope 10B is responsible for the majority of the neutron absorption. Boron-containing materials can also be used as neutron shielding, to reduce the activation of material close to a reactor core.
See also
* Nuclear power * Nuclear reactor *Nuclear safety
Nuclear safety is defined by the International Atomic Energy Agency (IAEA) as "The achievement of proper operating conditions, prevention of accidents or mitigation of accident consequences, resulting in protection of workers, the public and the ...
*Wigner effect
The Wigner effect (named for its discoverer, Eugene Wigner), also known as the discomposition effect or Wigner's disease, is the displacement of atoms in a solid caused by neutron radiation.
Any solid can display the Wigner effect. The effect is ...
References
External links
Further reading
* * * {{Authority control Alloys Nuclear power plant components Nuclear reactor safety Pressurized water reactors