Cleavage (chemistry) on:
[Wikipedia]
[Google]
[Amazon]
In
 In homolytic cleavage, or homolysis, the two electrons in a cleaved
In homolytic cleavage, or homolysis, the two electrons in a cleaved
 In heterolytic cleavage, or heterolysis, the bond breaks in such a fashion that the originally-
In heterolytic cleavage, or heterolysis, the bond breaks in such a fashion that the originally-
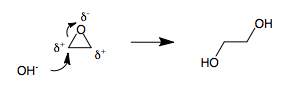 In a ring-opening, the cleaved molecule remains as a single unit. The bond breaks, but the two fragments remain attached by other parts of the structure. For example, an
In a ring-opening, the cleaved molecule remains as a single unit. The bond breaks, but the two fragments remain attached by other parts of the structure. For example, an
chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, bond cleavage, or bond fission, is the splitting of chemical bond
A chemical bond is a lasting attraction between atoms or ions that enables the formation of molecules and crystals. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds, or through the sharing of ...
s. This can be generally referred to as dissociation
Dissociation, in the wide sense of the word, is an act of disuniting or separating a complex object into parts. Dissociation may also refer to:
* Dissociation (chemistry), general process in which molecules or ionic compounds (complexes, or salts) ...
when a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
is cleaved into two or more fragments.
In general, there are two classifications for bond cleavage: ''homo''lytic and ''hetero''lytic, depending on the nature of the process. The triplet and singlet excitation energies of a sigma bond
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of s ...
can be used to determine if a bond will follow the homolytic or heterolytic pathway. A metal−metal sigma bond is an exception because the bond's excitation energy is extremely high, thus cannot be used for observation purposes.
In some cases, bond cleavage requires catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. Due to the high bond-dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
of C-H bonds, around , a large amount of energy is required to cleave the hydrogen atom from the carbon and bond a different atom to the carbon.
Homolytic cleavage
covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
are divided equally between the products. This process is also known as ''homolytic fission'' or ''radical fission''. The bond-dissociation energy of a bond is the amount of energy required to cleave the bond homolytically. This enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
change is one measure of bond strength
In chemistry, bond energy (''BE''), also called the mean bond enthalpy or average bond enthalpy is the measure of bond strength in a chemical bond. IUPAC defines bond energy as the average value of the gas-phase bond-dissociation energy (usually a ...
.
The triplet excitation energy of a sigma bond is the energy required for homolytic dissociation, but the actual excitation energy may be higher than the bond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
due to the repulsion between electrons in the triplet state
In quantum mechanics, a triplet is a quantum state of a system with a spin of quantum number =1, such that there are three allowed values of the spin component, = −1, 0, and +1.
Spin, in the context of quantum mechanics, is not a mechanical ...
.
Heterolytic cleavage
shared pair
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have no kn ...
s remain with one of the fragments. Thus, a fragment gains an electron, having both bonding electrons, while the other fragment loses an electron. This process is also known as ionic fission.
The singlet excitation energy of a sigma bond is the energy required for heterolytic dissociation, but the actual singlet excitation energy may be lower than the bond dissociation energy of heterolysis as a result of the Coulombic attraction
Electrostatics is a branch of physics that studies electric charges at Rest (physics), rest (static electricity).
Since classical antiquity, classical times, it has been known that some materials, such as amber, attract lightweight particles af ...
between the two ion fragments. The singlet excitation energy of a silicon–silicon sigma bond is lower than the carbon–carbon sigma bond, even though their bond strengths are 80kJ/mol and 70kJ/mol respectively, because silicon has higher electron affinity
The electron affinity (''E''ea) of an atom or molecule is defined as the amount of energy released when an electron attaches to a neutral atom or molecule in the gaseous state to form an anion.
::X(g) + e− → X−(g) + energy
Note that this is ...
and lower ionization potential
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule i ...
than carbon.
Heterolysis occurs naturally in reactions that involve electron donor
In chemistry, an electron donor is a chemical entity that donates electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process.
Typical reducing agents undergo permanent chem ...
ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
and transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
s which have empty orbitals.
Ring-opening
 In a ring-opening, the cleaved molecule remains as a single unit. The bond breaks, but the two fragments remain attached by other parts of the structure. For example, an
In a ring-opening, the cleaved molecule remains as a single unit. The bond breaks, but the two fragments remain attached by other parts of the structure. For example, an epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
ring can be opened by heterolytic cleavage of one of the polar carbon–oxygen bonds to give a single acyclic structure.
Applications
Inbiochemistry
Biochemistry or biological chemistry is the study of chemical processes within and relating to living organisms. A sub-discipline of both chemistry and biology, biochemistry may be divided into three fields: structural biology, enzymology and ...
, the process of breaking down large molecules by splitting their internal bonds is catabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, ...
. Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
s which catalyse bond cleavage are known as lyase
In biochemistry, a lyase is an enzyme that catalyzes the breaking (an elimination reaction) of various chemical bonds by means other than hydrolysis (a substitution reaction) and oxidation, often forming a new double bond or a new ring structure. ...
s, unless they operate by hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
or oxidoreduction, in which case they are known as hydrolase
Hydrolase is a class of enzyme that commonly perform as biochemical catalysts that use water to break a chemical bond, which typically results in dividing a larger molecule into smaller molecules. Some common examples of hydrolase enzymes are este ...
s and oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually ut ...
s respectively.
In proteomics
Proteomics is the large-scale study of proteins. Proteins are vital parts of living organisms, with many functions such as the formation of structural fibers of muscle tissue, enzymatic digestion of food, or synthesis and replication of DNA. In ...
, cleaving agents are used in proteome analysis where proteins are cleaved into smaller peptide fragments. Examples of cleaving agents used are cyanogen bromide
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. ...
, pepsin
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the gastric chief cells of the stomach lining and is one of the main digestive enzymes in the digestive systems of humans and many other animals, w ...
, and trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the dig ...
.
References
{{Authority control Chemical bonding