|
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Function In the duodenum, trypsin catalyzes the hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsinogen
Trypsinogen () is the precursor form (or zymogen) of trypsin, a digestive enzyme. It is produced by the pancreas and found in pancreatic juice, along with amylase, lipase, and chymotrypsinogen. It is cleaved to its active form, trypsin, by enteropeptidase, which is found in the intestinal mucosa. Once activated, the trypsin can cleave more trypsinogen into trypsin, a process called autoactivation. Trypsin cleaves the peptide bond on the carboxyl side of basic amino acids such as arginine and lysine. Function Trypsinogen is the proenzyme precursor of trypsin. Trypsinogen (the inactive form) is stored in the pancreas so that it may be released when required for protein digestion. The pancreas stores the inactive form trypsinogen because the active trypsin would cause severe damage to the tissue of the pancreas. Trypsinogen is released by the pancreas into the second part of the duodenum, via the pancreatic duct, along with other digestive enzymes. Activation of trypsinogen Tryps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Protease
Serine proteases (or serine endopeptidases) are enzymes that cleave peptide bonds in proteins. Serine serves as the nucleophilic amino acid at the (enzyme's) active site. They are found ubiquitously in both eukaryotes and prokaryotes. Serine proteases fall into two broad categories based on their structure: chymotrypsin-like (trypsin-like) or subtilisin-like. Classification The MEROPS protease classification system counts 16 superfamilies (as of 2013) each containing many families. Each superfamily uses the catalytic triad or dyad in a different protein fold and so represent convergent evolution of the catalytic mechanism. The majority belong to the S1 family of the PA clan (superfamily) of proteases. For superfamilies, P: superfamily, containing a mixture of nucleophile class families, S: purely serine proteases. superfamily. Within each superfamily, families are designated by their catalytic nucleophile, (S: serine proteases). Substrate specificity Ser ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enteropeptidase
Enteropeptidase (also called enterokinase) is an enzyme produced by cells of the duodenum and is involved in digestion in humans and other animals. Enteropeptidase converts trypsinogen (a zymogen) into its active form trypsin, resulting in the subsequent activation of pancreatic digestive enzymes. Absence of enteropeptidase results in intestinal digestion impairment. Enteropeptidase is a serine protease () consisting of a disulfide-linked heavy-chain of 82-140 kDa that anchors enterokinase in the intestinal brush border membrane and a light-chain of 35–62 kDa that contains the catalytic subunit. Enteropeptidase is a part of the chymotrypsin-clan of serine proteases, and is structurally similar to these proteins. Historical significance Enteropeptidase was discovered by Ivan Pavlov, who was awarded the 1904 Nobel Prize in Physiology or Medicine for his studies of gastrointestinal physiology. It is the first known enzyme to activate other enzymes, and it remains a remarkable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Triad
A catalytic triad is a set of three coordinated amino acids that can be found in the active site of some enzymes. Catalytic triads are most commonly found in hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An acid- base- nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to release the product and regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine or even selenocysteine. The 3D structure of the enzyme brings together the triad residues in a precise orientation, even though they may be far apart in the sequence ( primary structure). As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads sho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolysis
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolytic Cleavage
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PA Clan
The PA clan ( Proteases of mixed nucleophile, superfamily A) is the largest group of proteases with common ancestry as identified by structural homology. Members have a chymotrypsin-like fold and similar proteolysis mechanisms but can have identity of <10%. The clan contains both and (different ). PA clan proteases can be found in , |
Pancreas
The pancreas is an organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine gland, i.e. it has both an endocrine and a digestive exocrine function. 99% of the pancreas is exocrine and 1% is endocrine. As an endocrine gland, it functions mostly to regulate blood sugar levels, secreting the hormones insulin, glucagon, somatostatin, and pancreatic polypeptide. As a part of the digestive system, it functions as an exocrine gland secreting pancreatic juice into the duodenum through the pancreatic duct. This juice contains bicarbonate, which neutralizes acid entering the duodenum from the stomach; and digestive enzymes, which break down carbohydrates, proteins, and fats in food entering the duodenum from the stomach. Inflammation of the pancreas is known as pancreatitis, with common causes including chronic alcohol use and gallstone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zymogen
In biochemistry, a zymogen (), also called a proenzyme (), is an inactive precursor of an enzyme. A zymogen requires a biochemical change (such as a hydrolysis reaction revealing the active site, or changing the configuration to reveal the active site) for it to become an active enzyme. The biochemical change usually occurs in Golgi bodies, where a specific part of the precursor enzyme is cleaved in order to activate it. The inactivating piece which is cleaved off can be a peptide unit, or can be independently-folding domains comprising more than 100 residues. Although they limit the enzyme's ability, these N-terminal extensions of the enzyme or a “prosegment” often aid in the stabilization and folding of the enzyme they inhibit. The pancreas secretes zymogens partly to prevent the enzymes from digesting proteins in the cells in which they are synthesised. Enzymes like pepsin are created in the form of pepsinogen, an inactive zymogen. Pepsinogen is activated when chie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsinisation
Trypsinization is the process of cell dissociation using trypsin, a proteolytic enzyme which breaks down proteins, to dissociate adherent cells from the vessel in which they are being cultured. When added to a cell culture, trypsin breaks down the proteins that enable the cells to adhere to the vessel. Trypsinization is often used to pass cells to a new vessel. When the trypsinization process is complete the cells will be in suspension and appear rounded. For experimental purposes, cells are often cultivated in containers that take the form of plastic flasks or plates. In such flasks, cells are provided with growth medium comprising the essential nutrients required for proliferation, and the cells adhere to the container and each other as they grow. This process of cell culture or tissue culture requires a method to dissociate the cells from the container and each other. Trypsin, an enzyme commonly found in the digestive tract, can be used to "digest" the protein Proteins a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are Ribozyme, catalytic RNA molecules, called ribozymes. Enzymes' Chemical specificity, specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
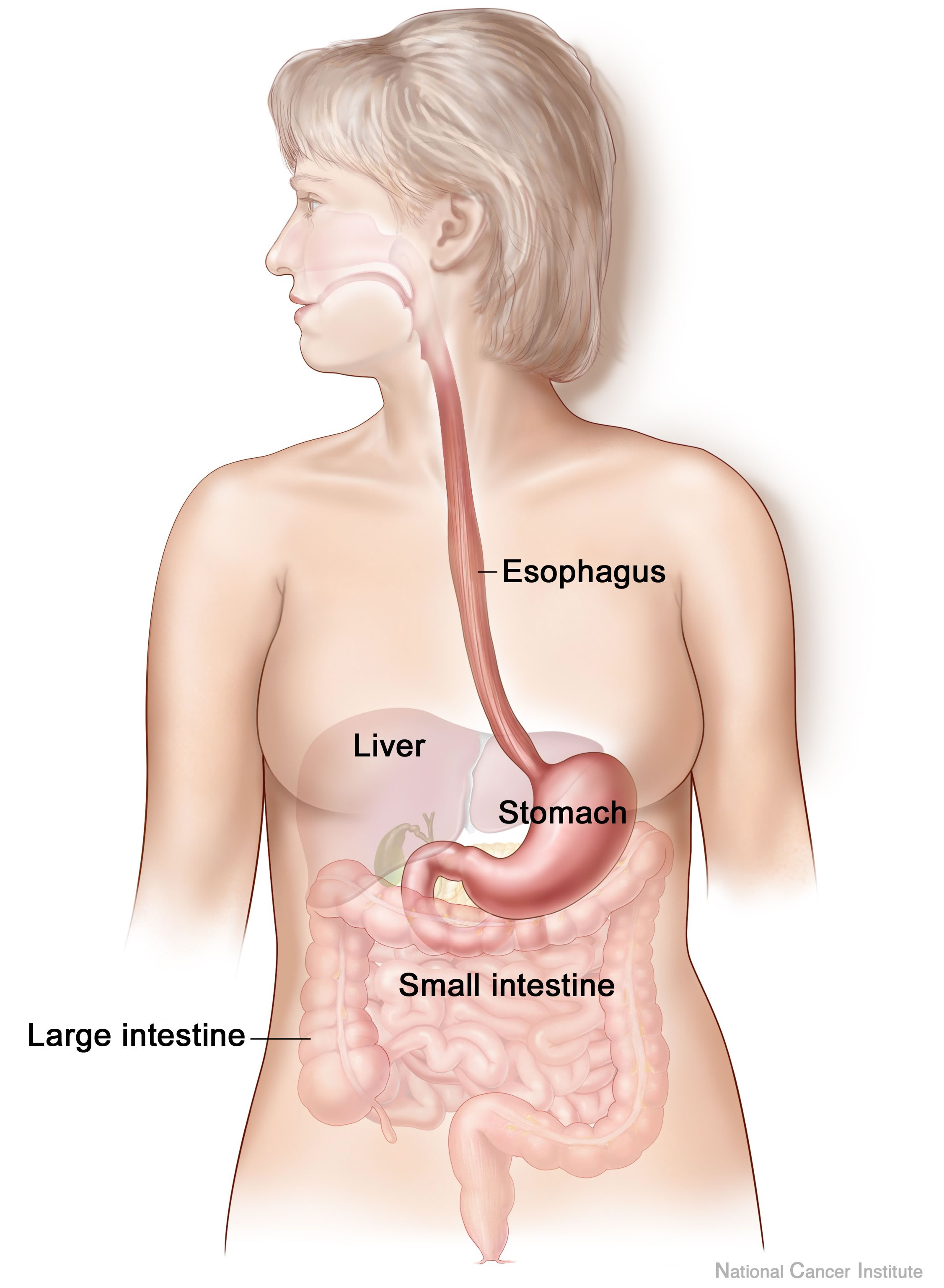
Digestive System
The human digestive system consists of the gastrointestinal tract plus the accessory organs of digestion (the tongue, salivary glands, pancreas, liver, and gallbladder). Digestion involves the breakdown of food into smaller and smaller components, until they can be absorbed and assimilated into the body. The process of digestion has three stages: the cephalic phase, the gastric phase, and the intestinal phase. The first stage, the cephalic phase of digestion, begins with secretions from gastric glands in response to the sight and smell of food. This stage includes the mechanical breakdown of food by chewing, and the chemical breakdown by digestive enzymes, that takes place in the mouth. Saliva contains the digestive enzymes amylase, and lingual lipase, secreted by the salivary and serous glands on the tongue. Chewing, in which the food is mixed with saliva, begins the mechanical process of digestion. This produces a bolus which is swallowed down the esophagus to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |