Chemical Polarity on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 A polar molecule has a net
A polar molecule has a net 
 In
In

 Examples of household nonpolar compounds include fats, oil, and petrol/gasoline. Most nonpolar molecules are water-insoluble (
Examples of household nonpolar compounds include fats, oil, and petrol/gasoline. Most nonpolar molecules are water-insoluble (
File:CHCA cleavable detergent.png, This amphiphilic molecule has several polar groups (
Chemical Bonding
Molecule Polarity
{{chemical bonds Physical chemistry Chemical properties Dimensionless numbers of chemistry
 In
In chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
, polarity is a separation of electric charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respe ...
leading to a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
or its chemical group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
s having an electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more polar bonds due to a difference in electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry.
Polar molecules interact through dipole–dipole intermolecular force
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles, e.g. a ...
s and hydrogen bonds
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
. Polarity underlies a number of physical properties including surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to f ...
, solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubil ...
, and melting and boiling points.
Polarity of bonds
Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called itselectronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
. Atoms with high electronegativitiessuch as fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reacti ...
, oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
, and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
exert a greater pull on electrons than atoms with lower electronegativities such as alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
s and alkaline earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are al ...
s. In a bond, this leads to unequal sharing of electrons between the atoms, as electrons will be drawn closer to the atom with the higher electronegativity.
Because electrons have a negative charge, the unequal sharing of electrons within a bond leads to the formation of an electric dipole
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The d ...
: a separation of positive and negative electric charge. Because the amount of charge separated in such dipoles is usually smaller than a fundamental charge
The elementary charge, usually denoted by is the electric charge carried by a single proton or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 . This elementary charge is a fundame ...
, they are called partial charges A partial charge is a non-integer charge value when measured in elementary charge units. Partial charge is more commonly called net atomic charge. It is represented by the Greek lowercase letter 𝛿, namely 𝛿− or 𝛿+.
Partial charges are c ...
, denoted as δ+ (delta
Delta commonly refers to:
* Delta (letter) (Δ or δ), a letter of the Greek alphabet
* River delta, at a river mouth
* D (NATO phonetic alphabet: "Delta")
* Delta Air Lines, US
* Delta variant of SARS-CoV-2 that causes COVID-19
Delta may also re ...
plus) and δ− (delta minus). These symbols were introduced by Sir Christopher Ingold and Dr. Edith Hilda (Usherwood) Ingold in 1926. The bond dipole moment is calculated by multiplying the amount of charge separated and the distance between the charges.
These dipoles within molecules can interact with dipoles in other molecules, creating dipole-dipole intermolecular forces.
Classification
Bonds can fall between one of two extremescompletely nonpolar or completely polar. A completely nonpolar bond occurs when the electronegativities are identical and therefore possess a difference of zero. A completely polar bond is more correctly called anionic bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. ...
, and occurs when the difference between electronegativities is large enough that one atom actually takes an electron from the other. The terms "polar" and "nonpolar" are usually applied to covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms ...
s, that is, bonds where the polarity is not complete. To determine the polarity of a covalent bond using numerical means, the difference between the electronegativity of the atoms is used.
Bond polarity is typically divided into three groups that are loosely based on the difference in electronegativity between the two bonded atoms. According to the Pauling scale
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
:
* ''Nonpolar bonds'' generally occur when the difference in electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
between the two atoms is less than 0.5
* ''Polar bonds'' generally occur when the difference in electronegativity between the two atoms is roughly between 0.5 and 2.0
* ''Ionic bonds
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. ...
'' generally occur when the difference in electronegativity between the two atoms is greater than 2.0
Pauling based this classification scheme on the ''partial ionic character'' of a bond, which is an approximate function of the difference in electronegativity between the two bonded atoms. He estimated that a difference of 1.7 corresponds to 50% ionic character, so that a greater difference corresponds to a bond which is predominantly ionic.
As a quantum-mechanical description, Pauling proposed that the wave function
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum system. The wave function is a complex-valued probability amplitude, and the probabilities for the possible results of measurements mad ...
for a polar molecule AB is a linear combination of wave functions for covalent and ionic molecules: ψ = aψ(A:B) + bψ(A+B−). The amount of covalent and ionic character depends on the values of the squared coefficients a2 and b2.
Bond dipole moments
The bond dipole moment uses the idea ofelectric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
to measure the polarity of a chemical bond within a molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
. It occurs whenever there is a separation of positive and negative charges.
The bond dipole μ is given by:
:.
The bond dipole is modeled as δ+ — δ– with a distance ''d'' between the partial charges A partial charge is a non-integer charge value when measured in elementary charge units. Partial charge is more commonly called net atomic charge. It is represented by the Greek lowercase letter 𝛿, namely 𝛿− or 𝛿+.
Partial charges are c ...
δ+ and δ–. It is a vector, parallel to the bond axis, pointing from minus to plus, as is conventional for electric dipole moment
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The ...
vectors.
Chemists often draw the vector pointing from plus to minus. This vector can be physically interpreted as the movement undergone by electrons when the two atoms are placed a distance ''d'' apart and allowed to interact, the electrons will move from their free state positions to be localised more around the more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
atom.
The SI unit
The International System of Units, known by the international abbreviation SI in all languages and sometimes pleonastically as the SI system, is the modern form of the metric system and the world's most widely used system of measurement. E ...
for electric dipole moment is the coulomb–meter. This is too large to be practical on the molecular scale.
Bond dipole moments are commonly measured in debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is give ...
s, represented by the symbol D, which is obtained by measuring the charge in units of 10−10 statcoulomb
The franklin (Fr) or statcoulomb (statC) electrostatic unit of charge (esu) is the physical unit for electrical charge used in the cgs-esu and Gaussian units. It is a derived unit given by
: 1 statC = 1 dyn1/2⋅cm = 1 cm3/2⋅g1/2⋅s−1.
That ...
and the distance ''d'' in Angstrom
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.m ...
s. Based on the conversion factor of
10−10 statcoulomb being 0.208 units of elementary charge, so 1.0 debye results from an electron and a proton separated by 0.208 Å. A useful conversion factor is 1 D = 3.335 64 C m.
For diatomic molecules there is only one (single or multiple) bond so the bond dipole moment is the molecular dipole moment, with typical values in the range of 0 to 11 D. At one extreme, a symmetrical molecule such as chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate betwee ...
, , has zero dipole moment, while near the other extreme, gas phase potassium bromide
Potassium bromide ( K Br) is a salt, widely used as an anticonvulsant and a sedative in the late 19th and early 20th centuries, with over-the-counter use extending to 1975 in the US. Its action is due to the bromide ion (sodium bromide is equall ...
, KBr, which is highly ionic, has a dipole moment of 10.41 D.
For polyatomic molecules, there is more than one bond. The total molecular dipole moment
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
may be approximated as the vector sum
In mathematics, physics, and engineering, a Euclidean vector or simply a vector (sometimes called a geometric vector or spatial vector) is a geometric object that has magnitude (or length) and direction. Vectors can be added to other vectors a ...
of the individual bond dipole moments. Often bond dipoles are obtained by the reverse process: a known total dipole of a molecule can be decomposed into bond dipoles. This is done to transfer bond dipole moments to molecules that have the same bonds, but for which the total dipole moment is not yet known. The vector sum of the transferred bond dipoles gives an estimate for the total (unknown) dipole of the molecule.
Polarity of molecules
While the molecules can be described as "polar covalent", "nonpolar covalent", or "ionic", this is often a relative term, with one molecule simply being ''more polar'' or ''more nonpolar'' than another. However, the following properties are typical of such molecules. A molecule is composed of one or more chemical bonds betweenmolecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
s of different atoms. A molecule may be polar either as a result of polar bonds due to differences in electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
as described above, or as a result of an asymmetric arrangement of nonpolar covalent bonds and non-bonding pairs of electrons known as a full molecular orbital
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding ...
.
Polar molecules
 A polar molecule has a net
A polar molecule has a net dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
as a result of the opposing charges (i.e. having partial positive and partial negative charges) from polar bonds arranged asymmetrically. Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a ...
(H2O) is an example of a polar molecule since it has a slight positive charge on one side and a slight negative charge on the other. The dipoles do not cancel out, resulting in a net dipole. Due to the polar nature of the water molecule itself, other polar molecules are generally able to dissolve in water. The dipole moment of water depends on its state. In the gas phase the dipole moment is ≈ 1.86 debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is give ...
(D), whereas liquid water (≈ 2.95 D) and ice (≈ 3.09 D) are higher due to differing hydrogen-bonded environments. Other examples include sugars (like sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
), which have many polar oxygen–hydrogen (−OH) groups and are overall highly polar.
If the bond dipole moments of the molecule do not cancel, the molecule is polar. For example, the water molecule
Water () is a polar inorganic compound that is at room temperature a tasteless and odorless liquid, which is nearly colorless apart from an inherent hint of blue. It is by far the most studied chemical compound and is described as the "uni ...
(H2O) contains two polar O−H bonds in a bent (nonlinear) geometry. The bond dipole moments do not cancel, so that the molecule forms a molecular dipole
In physics, a dipole () is an electromagnetic phenomenon which occurs in two ways:
*An electric dipole deals with the separation of the positive and negative electric charges found in any electromagnetic system. A simple example of this system i ...
with its negative pole at the oxygen and its positive pole midway between the two hydrogen atoms. In the figure each bond joins the central O atom with a negative charge (red) to an H atom with a positive charge (blue).
The hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock i ...
, HF, molecule is polar by virtue of polar covalent bondsin the covalent bond electrons are displaced toward the more electronegative fluorine atom.

Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous was ...
, NH3, is a molecule whose three N−H bonds have only a slight polarity (toward the more electronegative nitrogen atom). The molecule has two lone electrons in an orbital that points towards the fourth apex of an approximately regular tetrahedron, as predicted by the VSEPR theory
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theo ...
. This orbital is not participating in covalent bonding; it is electron-rich, which results in a powerful dipole across the whole ammonia molecule.
 In
In ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
(O3) molecules, the two O−O bonds are nonpolar (there is no electronegativity difference between atoms of the same element). However, the distribution of other electrons is unevensince the central atom has to share electrons with two other atoms, but each of the outer atoms has to share electrons with only one other atom, the central atom is more deprived of electrons than the others (the central atom has a formal charge
In chemistry, a formal charge (F.C. or q), in the covalent view of chemical bonding, is the charge assigned to an atom in a molecule, assuming that electrons in all chemical bonds are shared equally between atoms, regardless of relative electroneg ...
of +1, while the outer atoms each have a formal charge of −). Since the molecule has a bent geometry, the result is a dipole across the whole ozone molecule.
When comparing a polar and nonpolar molecule with similar molar masses, the polar molecule in general has a higher boiling point, because the dipole–dipole interaction between polar molecules results in stronger intermolecular attractions. One common form of polar interaction is the hydrogen bond
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a ...
, which is also known as the H-bond. For example, water forms H-bonds and has a molar mass M = 18 and a boiling point of +100 °C, compared to nonpolar methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
with M = 16 and a boiling point of –161 °C.
Nonpolar molecules
A molecule may be nonpolar either when there is an equal sharing of electrons between the two atoms of a diatomic molecule or because of the symmetrical arrangement of polar bonds in a more complex molecule. For example,boron trifluoride
Boron trifluoride is the inorganic compound with the formula BF3. This pungent, colourless, and toxic gas forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds.
Structure and bondin ...
(BF3) has a trigonal planar arrangement of three polar bonds at 120°. This results in no overall dipole in the molecule.


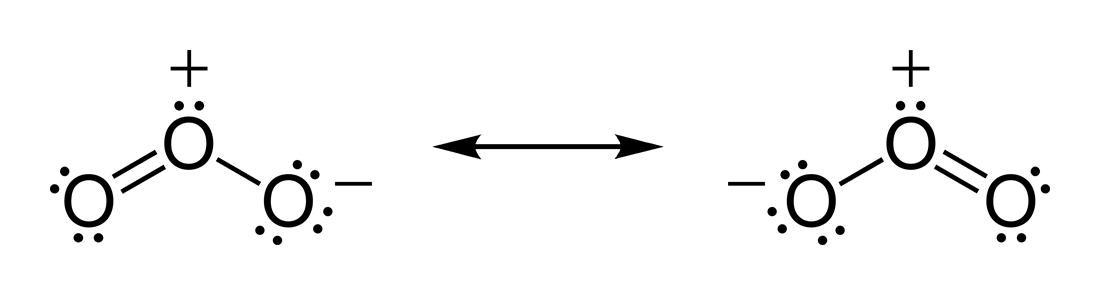
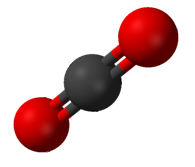
Carbon dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transpar ...
(CO2) has two polar C=O bonds, but the geometry of CO2 is linear so that the two bond dipole moments cancel and there is no net molecular dipole moment; the molecule is nonpolar.
 Examples of household nonpolar compounds include fats, oil, and petrol/gasoline. Most nonpolar molecules are water-insoluble (
Examples of household nonpolar compounds include fats, oil, and petrol/gasoline. Most nonpolar molecules are water-insoluble (hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
) at room temperature. Many nonpolar organic solvent
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for p ...
s, such as turpentine
Turpentine (which is also called spirit of turpentine, oil of turpentine, terebenthene, terebinthine and (colloquially) turps) is a fluid obtained by the distillation of resin harvested from living trees, mainly pines. Mainly used as a special ...
, are able to dissolve non-polar substances.
In the methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Eart ...
molecule (CH4) the four C−H bonds are arranged tetrahedrally around the carbon atom. Each bond has polarity (though not very strong). The bonds are arranged symmetrically so there is no overall dipole in the molecule. The diatomic oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as wel ...
molecule (O2) does not have polarity in the covalent bond because of equal electronegativity, hence there is no polarity in the molecule.
Amphiphilic molecules
Large molecules that have one end with polar groups attached and another end with nonpolar groups are described asamphiphile
An amphiphile (from the Greek αμφις amphis, both, and φιλíα philia, love, friendship), or amphipath, is a chemical compound possessing both hydrophilic (''water-loving'', polar) and lipophilic (''fat-loving'') properties. Such a compoun ...
s or ''amphiphilic'' molecules. They are good surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming ...
s and can aid in the formation of stable emulsions, or blends, of water and fats. Surfactants reduce the interfacial tension between oil and water by adsorbing at the liquid–liquid interface.
hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
, ''water-loving'') on the right side and a long nonpolar chain (lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipop ...
, ''fat-loving'') at the left side. This gives it surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming ...
properties
File:Micelle scheme-en.svg, A micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated collo ...
the lipophilic
Lipophilicity (from Greek λίπος "fat" and φίλος "friendly"), refers to the ability of a chemical compound to dissolve in fats, oils, lipids, and non-polar solvents such as hexane or toluene. Such non-polar solvents are themselves lipop ...
ends of the surfactant molecules dissolve in the oil, while the hydrophilic
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press.
In contrast, hydrophobes are no ...
charged ends remain outside in the water phase, shielding the rest of the hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
micelle. In this way, the small oil droplet becomes water-soluble.
File:Phospholipid schematic representation.png, Phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s are effective natural surfactants that have important biological functions
File:Phospholipids aqueous solution structures.svg, Cross section view of the structures that can be formed by phospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s. They can form a micelle
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated collo ...
and are vital in forming cell membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment ( ...
s
Predicting molecule polarity
Determining thepoint group
In geometry, a point group is a mathematical group of symmetry operations (isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every p ...
is a useful way to predict polarity of a molecule. In general, a molecule will not possess dipole moment if the individual bond dipole moments of the molecule cancel each other out. This is because dipole moments are euclidean vector
In mathematics, physics, and engineering, a Euclidean vector or simply a vector (sometimes called a geometric vector or spatial vector) is a geometric object that has magnitude (or length) and direction. Vectors can be added to other vectors ac ...
quantities with magnitude and direction, and a two equal vectors who oppose each other will cancel out.
Any molecule with a centre of inversion ("i") or a horizontal mirror plane ("σh") will not possess dipole moments.
Likewise, a molecule with more than one C''n'' axis of rotation will not possess a dipole moment because dipole moments cannot lie in more than one dimension
In physics and mathematics, the dimension of a Space (mathematics), mathematical space (or object) is informally defined as the minimum number of coordinates needed to specify any Point (geometry), point within it. Thus, a Line (geometry), lin ...
. As a consequence of that constraint, all molecules with dihedral symmetry
In mathematics, a dihedral group is the group of symmetries of a regular polygon, which includes rotations and reflections. Dihedral groups are among the simplest examples of finite groups, and they play an important role in group theory, ge ...
(D''n'') will not have a dipole moment because, by definition, D point groups have two or multiple C''n'' axes.
Since C1, Cs,C∞h C''n'' and C''n''v point groups
In geometry, a point group is a mathematical group of symmetry operations (isometries in a Euclidean space) that have a fixed point in common. The coordinate origin of the Euclidean space is conventionally taken to be a fixed point, and every p ...
do not have a centre of inversion, horizontal mirror planes or multiple C''n'' axis, molecules in one of those point groups will have dipole moment.
Electrical deflection of water
Contrary to popular misconception, the electrical deflection of a stream of water from a charged object is not based on polarity. The deflection occurs because of electrically charged droplets in the stream, which the charged object induces. A stream of water can also be deflected in a uniform electrical field, which cannot exert force on polar molecules. Additionally, after a stream of water is grounded, it can no longer be deflected. Weak deflection is even possible for nonpolar liquids.See also
*Chemical properties
A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity.William L. Masterton, Cecile N. Hurley ...
* Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
* Detergent
A detergent is a surfactant or a mixture of surfactants with cleansing properties when in dilute solutions. There are a large variety of detergents, a common family being the alkylbenzene sulfonates, which are soap-like compounds that are more ...
* Electronegativities of the elements (data page)
Electronegativity (Pauling scale)
Notes
* Separate values for each source are only given where one or more sources differ.
* Electronegativity is not a uniquely defined property and may depend on the definition. The suggested values are all ...
* Polar point group In geometry, a polar point group is a point group in which there is more than one point that every symmetry operation leaves unmoved. The unmoved points will constitute a line, a plane, or all of space.
While the simplest point group, C1, leaves a ...
References
External links
Chemical Bonding
Molecule Polarity
{{chemical bonds Physical chemistry Chemical properties Dimensionless numbers of chemistry