Capillary Effect on:
[Wikipedia]
[Google]
[Amazon]
 Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a
 Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capillary effect, or wicking) is the process of a liquid
A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of pressure. As such, it is one of the four fundamental states of matter (the others being solid, gas, a ...
flowing in a narrow space without the assistance of, or even in opposition to, any external forces like gravity
In physics, gravity () is a fundamental interaction which causes mutual attraction between all things with mass or energy. Gravity is, by far, the weakest of the four fundamental interactions, approximately 1038 times weaker than the stro ...
. The effect can be seen in the drawing up of liquids between the hairs of a paint-brush, in a thin tube, in porous materials such as paper and plaster, in some non-porous materials such as sand and liquefied carbon fiber
Carbon fiber-reinforced polymers (American English), carbon-fibre-reinforced polymers (Commonwealth English), carbon-fiber-reinforced plastics, carbon-fiber reinforced-thermoplastic (CFRP, CRP, CFRTP), also known as carbon fiber, carbon compo ...
, or in a biological cell. It occurs because of intermolecular forces between the liquid and surrounding solid surfaces. If the diameter of the tube is sufficiently small, then the combination of surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to f ...
(which is caused by cohesion within the liquid) and adhesive forces between the liquid and container wall act to propel the liquid.
Etymology
Capillary comes from the Latin word capillaris, meaning "of or resembling hair." The meaning stems from the tiny, hairlike diameter of a capillary. While capillary is usually used as a noun, the word also is used as an adjective, as in "capillary action," in which a liquid is moved along — even upward, against gravity — as the liquid is attracted to the internal surface of the capillaries.History
The first recorded observation of capillary action was byLeonardo da Vinci
Leonardo di ser Piero da Vinci (15 April 14522 May 1519) was an Italian polymath of the High Renaissance who was active as a painter, Drawing, draughtsman, engineer, scientist, theorist, sculptor, and architect. While his fame initially res ...
. A former student of Galileo
Galileo di Vincenzo Bonaiuti de' Galilei (15 February 1564 – 8 January 1642) was an Italian astronomer, physicist and engineer, sometimes described as a polymath. Commonly referred to as Galileo, his name was pronounced (, ). He was ...
, Niccolò Aggiunti, was said to have investigated capillary action. In 1660, capillary action was still a novelty to the Irish chemist Robert Boyle
Robert Boyle (; 25 January 1627 – 31 December 1691) was an Anglo-Irish natural philosopher, chemist, physicist, alchemist and inventor. Boyle is largely regarded today as the first modern chemist, and therefore one of the founders of ...
, when he reported that "some inquisitive French Men" had observed that when a capillary tube was dipped into water, the water would ascend to "some height in the Pipe". Boyle then reported an experiment in which he dipped a capillary tube into red wine and then subjected the tube to a partial vacuum. He found that the vacuum had no observable influence on the height of the liquid in the capillary, so the behavior of liquids in capillary tubes was due to some phenomenon different from that which governed mercury barometers.
Others soon followed Boyle's lead. Some (e.g., Honoré Fabri
Honoré Fabri (Honoratus Fabrius; 15 April 1608 – 8 March 1688) was a French Jesuit theologian, also known as ''Coningius''. He was a mathematician, physicist and controversialist.Jacob Bernoulli) thought that liquids rose in capillaries because air could not enter capillaries as easily as liquids, so the air pressure was lower inside capillaries. Others (e.g., Isaac Vossius, Giovanni Alfonso Borelli, Louis Carré, Francis Hauksbee, Josia Weitbrecht) thought that the particles of liquid were attracted to each other and to the walls of the capillary.
Although experimental studies continued during the 18th century, a successful quantitative treatment of capillary action was not attained until 1805 by two investigators: Thomas Young of the United Kingdom and

 Capillary penetration in porous media shares its dynamic mechanism with flow in hollow tubes, as both processes are resisted by viscous forces. Consequently, a common apparatus used to demonstrate the phenomenon is the ''capillary tube''. When the lower end of a glass tube is placed in a liquid, such as water, a concave
Capillary penetration in porous media shares its dynamic mechanism with flow in hollow tubes, as both processes are resisted by viscous forces. Consequently, a common apparatus used to demonstrate the phenomenon is the ''capillary tube''. When the lower end of a glass tube is placed in a liquid, such as water, a concave
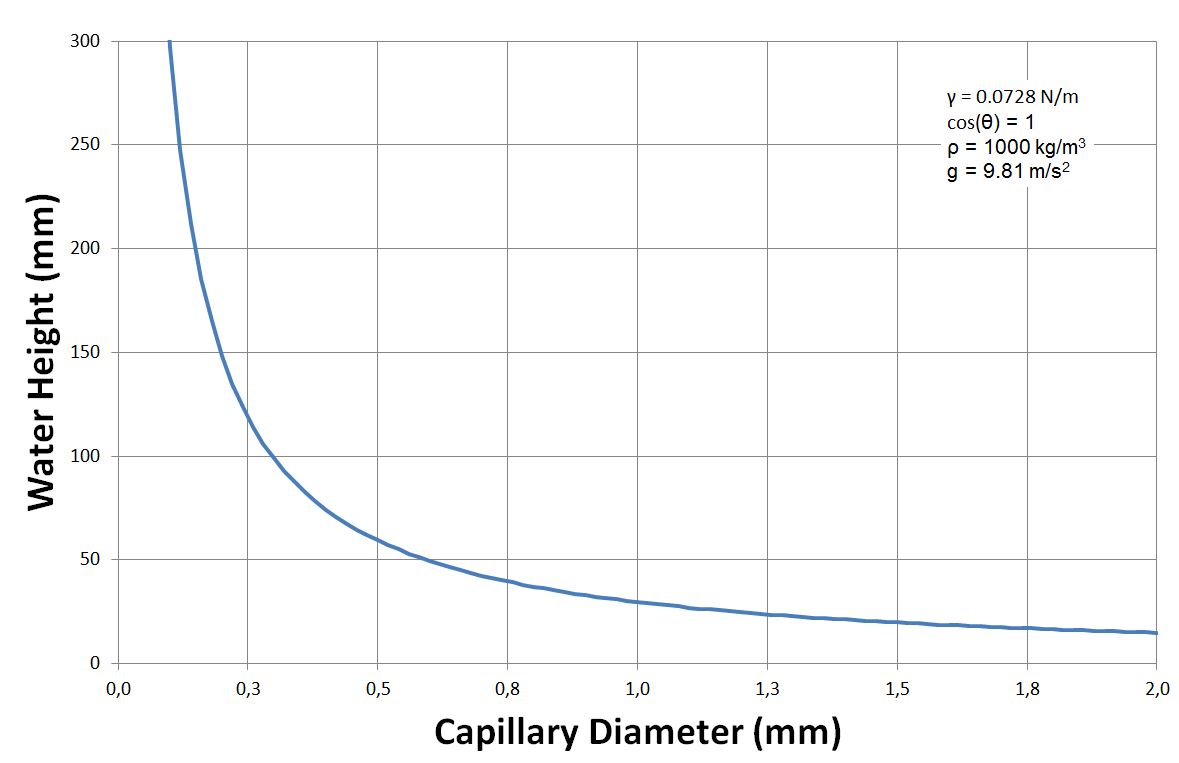 The height ''h'' of a liquid column is given by Jurin's law G.K. Batchelor, 'An Introduction To Fluid Dynamics', Cambridge University Press (1967) ,
:
where is the liquid-air
The height ''h'' of a liquid column is given by Jurin's law G.K. Batchelor, 'An Introduction To Fluid Dynamics', Cambridge University Press (1967) ,
:
where is the liquid-air
file:Kapilláris emelkedés 1.jpg
file:Kapilláris emelkedés 2.jpg
file:Kapilláris emelkedés 3.jpg
file:Kapilláris emelkedés 4.jpg
file:Kapilláris emelkedés 5.jpg
file:Kapilláris emelkedés 6.jpg
 When a dry porous medium is brought into contact with a liquid, it will absorb the liquid at a rate which decreases over time. When considering evaporation, liquid penetration will reach a limit dependent on parameters of temperature, humidity and permeability. This process is known as evaporation limited capillary penetration and is widely observed in common situations including fluid absorption into paper and rising damp in concrete or masonry walls. For a bar shaped section of material with cross-sectional area ''A'' that is wetted on one end, the cumulative volume ''V'' of absorbed liquid after a time ''t'' is
:
where ''S'' is the
When a dry porous medium is brought into contact with a liquid, it will absorb the liquid at a rate which decreases over time. When considering evaporation, liquid penetration will reach a limit dependent on parameters of temperature, humidity and permeability. This process is known as evaporation limited capillary penetration and is widely observed in common situations including fluid absorption into paper and rising damp in concrete or masonry walls. For a bar shaped section of material with cross-sectional area ''A'' that is wetted on one end, the cumulative volume ''V'' of absorbed liquid after a time ''t'' is
:
where ''S'' is the
page 131 on Google books
The above description is for the case where gravity and evaporation do not play a role. Sorptivity is a relevant property of building materials, because it affects the amount of rising dampness. Some values for the sorptivity of building materials are in the table below.
Pierre-Simon Laplace
Pierre-Simon, marquis de Laplace (; ; 23 March 1749 – 5 March 1827) was a French scholar and polymath whose work was important to the development of engineering, mathematics, statistics, physics, astronomy, and philosophy. He summarized ...
of France. They derived the Young–Laplace equation
In physics, the Young–Laplace equation () is an algebraic equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or ...
of capillary action. By 1830, the German mathematician Carl Friedrich Gauss had determined the boundary conditions governing capillary action (i.e., the conditions at the liquid-solid interface). In 1871, the British physicist Sir William Thomson
William Thomson, 1st Baron Kelvin, (26 June 182417 December 1907) was a British mathematician, mathematical physicist and engineer born in Belfast. Professor of Natural Philosophy at the University of Glasgow for 53 years, he did important ...
(later Lord Kelvin) determined the effect of the meniscus
Meniscus may refer to:
*Meniscus (anatomy), crescent-shaped fibrocartilaginous structure that partly divides a joint cavity
*Meniscus (liquid)
The meniscus (plural: ''menisci'', from the Greek for "crescent") is the curve in the upper surface ...
on a liquid's vapor pressure
Vapor pressure (or vapour pressure in English-speaking countries other than the US; see spelling differences) or equilibrium vapor pressure is defined as the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases ...
—a relation known as the Kelvin equation
The Kelvin equation describes the change in vapour pressure due to a curved liquid–vapor interface, such as the surface of a droplet. The vapor pressure at a convex curved surface is higher than that at a flat surface. The Kelvin equation is de ...
. German physicist Franz Ernst Neumann
Franz Ernst Neumann (11 September 1798 – 23 May 1895) was a German mineralogist, physicist and mathematician.
Biography
Neumann was born in Joachimsthal, Margraviate of Brandenburg, near Berlin. In 1815 he interrupted his studies at Berlin to ...
(1798–1895) subsequently determined the interaction between two immiscible liquids.
Albert Einstein
Albert Einstein ( ; ; 14 March 1879 – 18 April 1955) was a German-born theoretical physicist, widely acknowledged to be one of the greatest and most influential physicists of all time. Einstein is best known for developing the theory ...
's first paper, which was submitted to '' Annalen der Physik'' in 1900, was on capillarity.
Phenomena and physics

 Capillary penetration in porous media shares its dynamic mechanism with flow in hollow tubes, as both processes are resisted by viscous forces. Consequently, a common apparatus used to demonstrate the phenomenon is the ''capillary tube''. When the lower end of a glass tube is placed in a liquid, such as water, a concave
Capillary penetration in porous media shares its dynamic mechanism with flow in hollow tubes, as both processes are resisted by viscous forces. Consequently, a common apparatus used to demonstrate the phenomenon is the ''capillary tube''. When the lower end of a glass tube is placed in a liquid, such as water, a concave meniscus
Meniscus may refer to:
*Meniscus (anatomy), crescent-shaped fibrocartilaginous structure that partly divides a joint cavity
*Meniscus (liquid)
The meniscus (plural: ''menisci'', from the Greek for "crescent") is the curve in the upper surface ...
forms. Adhesion
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another).
The forces that cause adhesion and cohesion can be ...
occurs between the fluid and the solid inner wall pulling the liquid column along until there is a sufficient mass of liquid for gravitational forces to overcome these intermolecular forces. The contact length (around the edge) between the top of the liquid column and the tube is proportional to the radius of the tube, while the weight of the liquid column is proportional to the square of the tube's radius. So, a narrow tube will draw a liquid column along further than a wider tube will, given that the inner water molecules cohere sufficiently to the outer ones.
Examples
In the built environment, evaporation limited capillary penetration is responsible for the phenomenon of rising damp inconcrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most wi ...
and masonry
Masonry is the building of structures from individual units, which are often laid in and bound together by mortar; the term ''masonry'' can also refer to the units themselves. The common materials of masonry construction are bricks, building ...
, while in industry and diagnostic medicine this phenomenon is increasingly being harnessed in the field of paper-based microfluidics Paper-based microfluidics are microfluidic devices that consist of a series of hydrophilic cellulose or nitrocellulose fibers that transport fluid from an inlet through the porous medium to a desired outlet or region of the device, by means of capi ...
.
In physiology, capillary action is essential for the drainage of continuously produced tear fluid from the eye. Two canaliculi of tiny diameter are present in the inner corner of the eyelid, also called the lacrimal ducts; their openings can be seen with the naked eye within the lacrymal sacs when the eyelids are everted.
Wicking is the absorption of a liquid by a material in the manner of a candle wick.
Paper towel
A paper towel is an absorbent, disposable towel made from paper. In Britain, paper towels for kitchen use are also known as kitchen rolls, kitchen paper, or kitchen towels. For home use, paper towels are usually sold in a roll of perforated shee ...
s absorb liquid through capillary action, allowing a fluid
In physics, a fluid is a liquid, gas, or other material that continuously deforms (''flows'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are substances which cannot resist any shear ...
to be transferred from a surface to the towel. The small pores of a sponge
Sponges, the members of the phylum Porifera (; meaning 'pore bearer'), are a basal animal clade as a sister of the diploblasts. They are multicellular organisms that have bodies full of pores and channels allowing water to circulate through t ...
act as small capillaries, causing it to absorb a large amount of fluid. Some textile fabrics are said to use capillary action to "wick" sweat away from the skin. These are often referred to as wicking fabrics, after the capillary properties of candle
A candle is an ignitable wick embedded in wax, or another flammable solid substance such as tallow, that provides light, and in some cases, a fragrance. A candle can also provide heat or a method of keeping time.
A person who makes candles i ...
and lamp wicks.
Capillary action is observed in thin layer chromatography, in which a solvent moves vertically up a plate via capillary action. In this case the pores are gaps between very small particles.
Capillary action draws ink
Ink is a gel, sol, or solution that contains at least one colorant, such as a dye or pigment, and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, reed pen, or quill. Thi ...
to the tips of fountain pen
A fountain pen is a writing instrument which uses a metal nib to apply a water-based ink to paper. It is distinguished from earlier dip pens by using an internal reservoir to hold ink, eliminating the need to repeatedly dip the pen in an inkw ...
nibs from a reservoir or cartridge inside the pen.
With some pairs of materials, such as mercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
and glass, the intermolecular forces within the liquid exceed those between the solid and the liquid, so a convex meniscus forms and capillary action works in reverse.
In hydrology
Hydrology () is the scientific study of the movement, distribution, and management of water on Earth and other planets, including the water cycle, water resources, and environmental watershed sustainability. A practitioner of hydrology is calle ...
, capillary action describes the attraction of water molecules to soil particles. Capillary action is responsible for moving groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidate ...
from wet areas of the soil to dry areas. Differences in soil potential () drive capillary action in soil.
A practical application of capillary action is the capillary action siphon. Instead of utilizing a hollow tube (as in most siphons), this device consists of a length of cord made of a fibrous material (cotton cord or string works well). After saturating the cord with water, one (weighted) end is placed in a reservoir full of water, and the other end placed in a receiving vessel. The reservoir must be higher than the receiving vessel. A related but simplified capillary siphon only consists of two hook-shaped stainless-steel rods, whose surface is hydrophilic, allowing water to wet the narrow grooves between them. Due to capillary action and gravity, water will slowly transfer from the reservoir to the receiving vessel. This simple device can be used to water houseplants when nobody is home. This property is also made use of in the lubrication of steam locomotives: wicks of worsted wool
Worsted ( or ) is a high-quality type of wool yarn, the fabric made from this yarn, and a yarn weight category. The name derives from Worstead, a village in the English county of Norfolk. That village, together with North Walsham and Aylsham, for ...
are used to draw oil from reservoirs into delivery pipes leading to the bearings.
In plants and animals
Capillary action is seen in many plants, and plays a part intranspiration
Transpiration is the process of water movement through a plant and its evaporation from aerial parts, such as leaves, stems and flowers. Water is necessary for plants but only a small amount of water taken up by the roots is used for growth a ...
. Water is brought high up in trees by branching; evaporation at the leaves creating depressurization; probably by osmotic pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane.
It is also defined as the measure of the tendency of a solution to take in a pure ...
added at the roots; and possibly at other locations inside the plant, especially when gathering humidity with air root
Aerial roots are roots above the ground. They are almost always adventitious. They are found in diverse plant species, including epiphytes such as orchids (''Orchidaceae''), tropical coastal swamp trees such as mangroves, banyan figs ('' F ...
s.
Capillary action for uptake of water has been described in some small animals, such as ''Ligia exotica
''Ligia exotica'', also called sea roach or wharf roach, is a woodlouse-like isopod, a sea slater in the family Ligiidae. It is found in various parts of the world living on rocky coasts and harbour walls just above high water mark.
Descripti ...
'' and '' Moloch horridus''.
Height of a meniscus
Capillary rise of liquid in a capillary
 The height ''h'' of a liquid column is given by Jurin's law G.K. Batchelor, 'An Introduction To Fluid Dynamics', Cambridge University Press (1967) ,
:
where is the liquid-air
The height ''h'' of a liquid column is given by Jurin's law G.K. Batchelor, 'An Introduction To Fluid Dynamics', Cambridge University Press (1967) ,
:
where is the liquid-air surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to f ...
(force/unit length), ''θ'' is the contact angle, ''ρ'' is the density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematical ...
of liquid (mass/volume), ''g'' is the local acceleration due to gravity (length/square of time), and ''r'' is the radius
In classical geometry, a radius ( : radii) of a circle or sphere is any of the line segments from its center to its perimeter, and in more modern usage, it is also their length. The name comes from the latin ''radius'', meaning ray but also the ...
of tube.
As ''r'' is in the denominator, the thinner the space in which the liquid can travel, the further up it goes. Likewise, lighter liquid and lower gravity increase the height of the column.
For a water-filled glass tube in air at standard laboratory conditions, at 20°C, , and . Because water spreads on clean glass, the effective equilibrium contact angle is approximately zero. For these values, the height of the water column is
:
Thus for a radius glass tube in lab conditions given above, the water would rise an unnoticeable . However, for a radius tube, the water would rise , and for a radius tube, the water would rise .
Capillary rise of liquid between two glass plates
The product of layer thickness (''d'') and elevation height (''h'') is constant (''d''·''h'' = constant), the two quantities are inversely proportional. The surface of the liquid between the planes ishyperbola
In mathematics, a hyperbola (; pl. hyperbolas or hyperbolae ; adj. hyperbolic ) is a type of smooth curve lying in a plane, defined by its geometric properties or by equations for which it is the solution set. A hyperbola has two pieces, cal ...
.
Liquid transport in porous media
 When a dry porous medium is brought into contact with a liquid, it will absorb the liquid at a rate which decreases over time. When considering evaporation, liquid penetration will reach a limit dependent on parameters of temperature, humidity and permeability. This process is known as evaporation limited capillary penetration and is widely observed in common situations including fluid absorption into paper and rising damp in concrete or masonry walls. For a bar shaped section of material with cross-sectional area ''A'' that is wetted on one end, the cumulative volume ''V'' of absorbed liquid after a time ''t'' is
:
where ''S'' is the
When a dry porous medium is brought into contact with a liquid, it will absorb the liquid at a rate which decreases over time. When considering evaporation, liquid penetration will reach a limit dependent on parameters of temperature, humidity and permeability. This process is known as evaporation limited capillary penetration and is widely observed in common situations including fluid absorption into paper and rising damp in concrete or masonry walls. For a bar shaped section of material with cross-sectional area ''A'' that is wetted on one end, the cumulative volume ''V'' of absorbed liquid after a time ''t'' is
:
where ''S'' is the sorptivity
In 1957 John Philip introduced the term sorptivity and defined it as ''a measure of the capacity of the medium to absorb or desorb liquid by capillarity
Capillary action (sometimes called capillarity, capillary motion, capillary rise, capi ...
of the medium, in units of m·s−1/2 or mm·min−1/2. This time dependence relation is similar to Washburn's equation for the wicking in capillaries and porous media. The quantity
:
is called the cumulative liquid intake, with the dimension of length. The wetted length of the bar, that is the distance between the wetted end of the bar and the so-called ''wet front'', is dependent on the fraction ''f'' of the volume occupied by voids. This number ''f'' is the porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0% and 100%. Strictly speaking, some tests measure ...
of the medium; the wetted length is then
:
Some authors use the quantity ''S/f'' as the sorptivity.C. Hall, W.D. Hoff, Water transport in brick, stone, and concrete. (2002page 131 on Google books
The above description is for the case where gravity and evaporation do not play a role. Sorptivity is a relevant property of building materials, because it affects the amount of rising dampness. Some values for the sorptivity of building materials are in the table below.
See also
*Bond number
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
* Bound water
* Capillary fringe
The capillary fringe is the subsurface layer in which groundwater seeps up from a water table by capillary action to fill pores. Pores at the base of the capillary fringe are filled with water due to tension saturation. This saturated portion of t ...
* Capillary pressure In fluid statics, capillary pressure () is the pressure between two immiscible fluids in a thin tube (see capillary action), resulting from the interactions of forces between the fluids and solid walls of the tube. Capillary pressure can serve as bo ...
* Capillary wave
* Capillary bridges Usually, we understand the term capillary bridge as a minimized surface of liquid or membrane, created between two rigid bodies with an arbitrary shape. Capillary bridges also may form between two liquids. Plateau defined a sequence of capillary s ...
* Damp-proof course
Damp proofing in construction is a type of moisture control applied to building walls and floors to prevent moisture from passing into the interior spaces. Dampness problems are among the most frequent problems encountered in residences.
''Damp p ...
* Darcy's law
* Frost flowers
A frost flower or ice flower is formed when thin layers of ice are extruded from long-stemmed plants in autumn or early winter. The thin layers of ice are often formed into exquisite patterns that curl into "petals" that resemble flowers.
Typ ...
* Frost heaving
* Hindu milk miracle
* Krogh model
Krogh model is a scientific model of mass transfer explaining the concentration of molecular oxygen through a cylindrical capillary tube as a function of a changing position over the capillary tube's length. It was first conceptualized by August K ...
* Mercury intrusion porosimetry
* Needle ice
Needle ice is a needle-shaped column of ice formed by groundwater. Needle ice forms when the temperature of the soil is above and the surface temperature of the air is below . Liquid water underground rises to the surface by capillary action, a ...
* Surface tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension is what allows objects with a higher density than water such as razor blades and insects (e.g. water striders) to f ...
* Washburn's equation
* Young–Laplace equation
In physics, the Young–Laplace equation () is an algebraic equation that describes the capillary pressure difference sustained across the interface between two static fluids, such as water and air, due to the phenomenon of surface tension or ...
References
Further reading
* {{Authority control Fluid dynamics Hydrology Surface science Porous media