Aminoacyl-tRNA Synthetase on:
[Wikipedia]
[Google]
[Amazon]
An aminoacyl-tRNA synthetase (aaRS or ARS), also called tRNA-ligase, is an
ICAARS
B. Pawar, and GPS Raghava (2010) Prediction and classification of aminoacyl tRNA synthetases using PROSITE domains
BMC Genomics 2010, 11:507MARSpred
*Prokaryotic AAR
database
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
that attaches the appropriate amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
onto its corresponding tRNA
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ...
. It does so by catalyzing the transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction ca ...
of a specific cognate amino acid or its precursor to one of all its compatible cognate tRNAs to form an aminoacyl-tRNA
Aminoacyl-tRNA (also aa-tRNA or charged tRNA) is tRNA to which its cognate amino acid is chemically bonded (charged). The aa-tRNA, along with particular elongation factors, deliver the amino acid to the ribosome for incorporation into the polypept ...
. In humans, the 20 different types of aa-tRNA are made by the 20 different aminoacyl-tRNA synthetases, one for each amino acid of the genetic code
The genetic code is the set of rules used by living cells to translate information encoded within genetic material ( DNA or RNA sequences of nucleotide triplets, or codons) into proteins. Translation is accomplished by the ribosome, which links ...
.
This is sometimes called "charging" or "loading" the tRNA with an amino acid. Once the tRNA is charged, a ribosome can transfer the amino acid from the tRNA onto a growing peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
...
, according to the genetic code. Aminoacyl tRNA therefore plays an important role in RNA translation
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
, the expression of genes to create proteins.
Mechanism
Thesynthetase
In biochemistry, a ligase is an enzyme that can catalyze the joining (ligation) of two large molecules by forming a new chemical bond. This is typically via hydrolysis of a small pendant chemical group on one of the larger molecules or the enz ...
first binds ATP and the corresponding amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha a ...
(or its precursor
Precursor or Precursors may refer to:
* Precursor (religion), a forerunner, predecessor
** The Precursor, John the Baptist
Science and technology
* Precursor (bird), a hypothesized genus of fossil birds that was composed of fossilized parts of u ...
) to form an aminoacyl-adenylate, releasing inorganic pyrophosphate (PPi). The adenylate-aaRS complex then binds the appropriate tRNA molecule's D arm
The D arm is a feature in the tertiary structure of transfer RNA (tRNA). It is composed of the two D stems and the D loop. The D loop contains the base dihydrouridine, for which the arm is named. The D loop's main function is that of recognition ...
, and the amino acid is transferred from the aa-AMP to either the 2'- or the 3'-OH of the last tRNA nucleotide (A76) at the 3'-end.
The mechanism can be summarized in the following reaction series:
# Amino Acid + ATP → Aminoacyl-AMP + PPi
# Aminoacyl-AMP + tRNA → Aminoacyl-tRNA + AMP #REDIRECT Amp
{{Redirect category shell, {{R from other capitalisation{{R from ambiguous page ...
Summing the reactions, the highly exergonic
An exergonic process is one which there is a positive flow of energy from the system to the surroundings. This is in contrast with an endergonic process. Constant pressure, constant temperature reactions are exergonic if and only if the Gibbs fr ...
overall reaction is as follows:
* Amino Acid + tRNA + ATP → Aminoacyl-tRNA + AMP + PPi
Some synthetases also mediate an editing reaction to ensure high fidelity of tRNA charging. If the incorrect tRNA is added (aka. the tRNA is found to be improperly charged), the aminoacyl-tRNA bond is hydrolyzed. This can happen when two amino acids have different properties even if they have similar shapes—as is the case with valine
Valine (symbol Val or V) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotona ...
and threonine.
The accuracy of aminoacyl-tRNA synthetase is so high that it is often paired with the word "superspecificity” when it is compared to other enzymes that are involved in metabolism. Although not all synthetases have a domain with the sole purpose of editing, they make up for it by having specific binding and activation of their affiliated amino acids. Another contribution to the accuracy of these synthetases is the ratio of concentrations of aminoacyl-tRNA synthetase and its cognate tRNA. Since tRNA synthetase improperly acylates the tRNA when the synthetase is overproduced, a limit must exist on the levels of aaRSs and tRNAs in vivo.
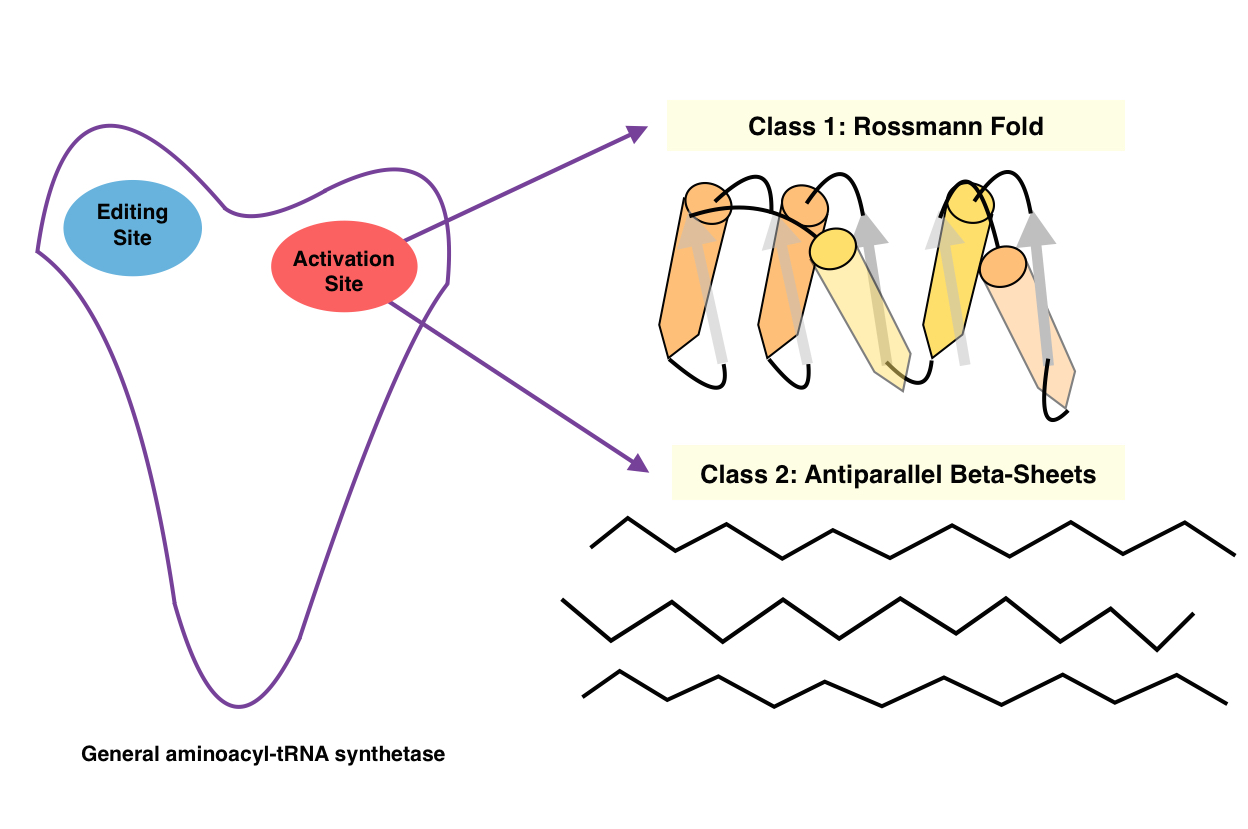
Classes
There are two classes of aminoacyl tRNA synthetase, each composed of ten enzymes: * Class I has two highly conserved sequence motifs. It aminoacylates at the 2'-OH of a terminal adenosinenucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecule ...
on tRNA, and it is usually monomeric
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
or dimeric (one or two subunits, respectively).
* Class II has three highly conserved sequence motifs. It aminoacylates at the 3'-OH of a terminal adenosine on tRNA, and is usually dimeric or tetramer
A tetramer () ('' tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula ...
ic (two or four subunits, respectively). Although phenylalanine-tRNA synthetase is class II, it aminoacylates at the 2'-OH.
The amino acids are attached to the hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydro ...
(-OH) group of the adenosine via the carboxyl
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
(-COOH) group.
Regardless of where the aminoacyl is initially attached to the nucleotide, the 2'-''O''-aminoacyl-tRNA will ultimately migrate to the 3' position via transesterification
In organic chemistry, transesterification is the process of exchanging the organic group R″ of an ester with the organic group R' of an alcohol. These reactions are often catalyzed by the addition of an acid or base catalyst. The reaction ca ...
.

Structures
Both classes of aminoacyl-tRNA synthetases are multidomain proteins. In a typical scenario, an aaRS consists of acatalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
domain (where both the above reactions take place) and an anticodon binding domain (which interacts mostly with the anticodon region of the tRNA). Transfer-RNAs for different amino acids differ not only in their anticodon but also at other points, giving them slightly different overall configurations. The aminoacyl-tRNA synthetases recognize the correct tRNAs primarily through their overall configuration, not just through their anticodon. In addition, some aaRSs have additional RNA binding domains and editing domains that cleave incorrectly paired aminoacyl-tRNA molecules.
The catalytic domains of all the aaRSs of a given class are found to be homologous to one another, whereas class I and class II aaRSs are unrelated to one another. The class I aaRSs feature a cytidylyltransferase-like Rossmann fold
The Rossmann fold is a tertiary fold found in proteins that bind nucleotides, such as enzyme cofactors FAD, NAD+, and NADP+. This fold is composed of alternating beta strands and alpha helical segments where the beta strands are hydrogen bonde ...
seen in proteins like glycerol-3-phosphate cytidylyltransferase, nicotinamide nucleotide adenylyltransferase and archaeal FAD synthase, whereas the class II aaRSs have a unique fold related to biotin and lipoate ligases.
The alpha helical
The alpha helix (α-helix) is a common motif in the secondary structure of proteins and is a right hand-helix conformation in which every backbone N−H group hydrogen bonds to the backbone C=O group of the amino acid located four residues ear ...
anticodon
Transfer RNA (abbreviated tRNA and formerly referred to as sRNA, for soluble RNA) is an adaptor molecule composed of RNA, typically 76 to 90 nucleotides in length (in eukaryotes), that serves as the physical link between the mRNA and the amino ...
binding domain of Arginyl, Glycyl and Cysteinyl-tRNA synthetases is known as the DALR domain after characteristic conserved amino acids.
Aminoacyl-tRNA synthetases have been kinetically studied, showing that Mg2+ ions play an active catalytic role and therefore aaRs have a degree of magnesium dependence. Increasing the Mg2+ concentration leads to an increase in the equilibrium constants for the aminoacyl-tRNA synthetases’ reactions. Although this trend was seen in both class I and class II synthetases, the magnesium dependence for the two classes are very distinct. Class II synthetases have two or three (more frequently three) Mg2+ ions, while class I only requires one Mg2+ ion.
Beside their lack of overall sequence and structure similarity, class I and class II synthetases feature different ATP recognition mechanisms. While class I binds via interactions mediated by backbone hydrogen bonds, class II uses a pair of arginine residues to establish salt bridges to its ATP ligand. This oppositional implementation is manifested in two structural motifs, the Backbone Brackets and Arginine Tweezers, which are observable in all class I and class II structures, respectively. The high structural conservation of these motifs suggest that they must have been present since ancient times.
Evolution
Most of the aaRSs of a given specificity are evolutionarily closer to one another than to aaRSs of another specificity. However, AsnRS and GlnRS group within AspRS and GluRS, respectively. Most of the aaRSs of a given specificity also belong to a single class. However, there are two distinct versions of the LysRS - one belonging to the class I family and the other belonging to the class II family. The molecular phylogenies of aaRSs are often not consistent with accepted organismalphylogenies
A phylogenetic tree (also phylogeny or evolutionary tree Felsenstein J. (2004). ''Inferring Phylogenies'' Sinauer Associates: Sunderland, MA.) is a branching diagram or a tree showing the evolutionary relationships among various biological spec ...
. That is, they violate the so-called canonical phylogenetic pattern shown by most other enzymes for the three domains of life - '' Archaea'', ''Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
'', and ''Eukarya
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bact ...
''. Furthermore, the phylogenies inferred for aaRSs of different amino acids often do not agree with one another. In addition, aaRS paralogs
Sequence homology is the biological homology between DNA, RNA, or protein sequences, defined in terms of shared ancestry in the evolutionary history of life. Two segments of DNA can have shared ancestry because of three phenomena: either a sp ...
within the same species show a high degree of divergence between them. These are clear indications that horizontal transfer has occurred several times during the evolutionary history of aaRSs.
A widespread belief in the evolutionary stability of this superfamily, meaning that every organism has all the aaRSs for their corresponding aminoacids, is misconceived. A large-scale genomic analysis on ~2500 prokaryotic genomes showed that many of them miss one or more aaRS genes whereas many genomes have 1 or more paralogs. AlaRS, GlyRS, LeuRS, IleRS and ValRS are the most evolutionarily stable members of the family. GluRS, LysRS and CysRS often have paralogs, whereas AsnRS, GlnRS, PylRS and SepRS are often absent from many genomes.
With the exception of AlaRS, it has been discovered that 19 out of the 20 human aaRSs have added at least one new domain or motif. These new domains and motifs vary in function and are observed in various forms of life. A common novel function within human aaRSs is providing additional regulation of biological processes. There exists a theory that the increasing number of aaRSs that add domains is due to the continuous evolution of higher organisms with more complex and efficient building blocks and biological mechanisms. One key piece of evidence to this theory is that after a new domain is added to an aaRS, the domain becomes fully integrated. This new domain's functionality is conserved from that point on.
As genetic efficiency evolved in higher organisms, 13 new domains with no obvious association with the catalytic activity of aaRSs genes have been added.
Application in biotechnology
In some of the aminoacyl tRNA synthetases, the cavity that holds the amino acid can be mutated and modified to carry unnatural amino acids synthesized in the lab, and to attach them to specific tRNAs. This expands the genetic code, beyond the twenty canonical amino acids found in nature, to include an unnatural amino acid as well. The unnatural amino acid is coded by a nonsense (TAG, TGA, TAA) triplet, a quadruplet codon, or in some cases a redundant rare codon. The organism that expresses the mutant synthetase can then be genetically programmed to incorporate the unnatural amino acid into any desired position in any protein of interest, allowing biochemists or structural biologists to probe or change the protein's function. For instance, one can start with the gene for a protein that binds a certain sequence of DNA, and, by directing an unnatural amino acid with a reactive side-chain into the binding site, create a new protein that cuts the DNA at the target-sequence, rather than binding it. By mutating aminoacyl tRNA synthetases, chemists have expanded the genetic codes of various organisms to include lab-synthesized amino acids with all kinds of useful properties: photoreactive, metal-chelating, xenon-chelating, crosslinking, spin-resonant, fluorescent, biotinylated, and redox-active amino acids. Another use is introducing amino acids bearing reactive functional groups for chemically modifying the target protein. Certain diseases’ causation (such as neuronal pathologies, cancer, disturbed metabolic conditions, and autoimmune disorders) have been correlated to specific mutations of aminoacyl-tRNA synthetases. Charcot-Marie-Tooth (CMT) is the most frequent heritable disorder of the peripheral nervous system (a neuronal disease) and is caused by a heritable mutation in glycol-tRNA and tyrosyl-tRNA. Diabetes, a metabolic disease, induces oxidative stress, which triggers a build up of mitochondrial tRNA mutations. It has also been discovered that tRNA synthetases may be partially involved in the etiology of cancer. A high level of expression or modification of aaRSs has been observed within a range of cancers. A common outcome from mutations of aaRSs is a disturbance of dimer shape/formation which has a direct relationship with its function. These correlations between aaRSs and certain diseases have opened up a new door to synthesizing therapeutics.Noncatalytic domains
The novel domain additions to aaRS genes are accretive and progressive up the ''Tree of Life
The tree of life is a fundamental archetype in many of the world's mythological, religious, and philosophical traditions. It is closely related to the concept of the sacred tree.Giovino, Mariana (2007). ''The Assyrian Sacred Tree: A Hist ...
''. The strong evolutionary pressure for these small non-catalytic protein domains suggested their importance. Findings beginning in 1999 and later revealed a previously unrecognized layer of biology: these proteins control gene expression within the cell of origin, and when released exert homeostatic and developmental control in specific human cell types, tissues and organs during adult or fetal development or both, including pathways associated with '' angiogenesis'', ''inflammation
Inflammation (from la, inflammatio) is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants, and is a protective response involving immune cells, blood vessels, and molec ...
'', the ''immune response
An immune response is a reaction which occurs within an organism for the purpose of defending against foreign invaders. These invaders include a wide variety of different microorganisms including viruses, bacteria, parasites, and fungi which could ...
'', the ''mechanistic target of rapamycin
The mammalian target of rapamycin (mTOR), also referred to as the mechanistic target of rapamycin, and sometimes called FK506-binding protein 12-rapamycin-associated protein 1 (FRAP1), is a kinase that in humans is encoded by the ''MTOR'' gene. ...
'' (mTOR) signalling, '' apoptosis'', ''tumorigenesis
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnor ...
'', and ''interferon gamma
Interferon gamma (IFN-γ) is a dimerized soluble cytokine that is the only member of the type II class of interferons. The existence of this interferon, which early in its history was known as immune interferon, was described by E. F. Wheeloc ...
'' (IFN-) and ''p53
p53, also known as Tumor protein P53, cellular tumor antigen p53 (UniProt name), or transformation-related protein 53 (TRP53) is a regulatory protein that is often mutated in human cancers. The p53 proteins (originally thought to be, and often s ...
'' signalling.
Substrate Depletion
In 2022, it was discovered that aminoacyl-trna synthetases may incorporate alternative amino acids during shortages of their precursors. In particular, tryptophanyl-tRNA synthetase ( WARS1) will incorporate phenylalanine during tryptophan depletion, essentially inducing a W>Fcodon reassignment Codon reassignment is the biological process via which the genetic code of a cell is changed as a response to the environment. It may be caused by alternative tRNA aminoacylation, in which the cell modifies the target aminoacid of some particular ...
. Depletion of the other substrate of aminoacyl-tRNA synthetases, the cognate tRNA, may be relevant to certain diseases, e.g. Charcot–Marie–Tooth disease
Charcot–Marie–Tooth disease (CMT) is a hereditary motor and sensory neuropathy of the peripheral nervous system characterized by progressive loss of muscle tissue and touch sensation across various parts of the body. This disease is the most ...
. It was shown that CMT-mutant glycyl-tRNA synthetase variants are still able to bind tRNA-gly but fail to release it, leading to depletion of the cellular pool of glycyl-tRNA-gly, what in turn results in stalling of the ribosome on glycine codons during mRNA translation.
Clinical
Mutations in the mitochondrial enzyme have been associated with a number of genetic disorders includingLeigh syndrome
Leigh syndrome (also called Leigh disease and subacute necrotizing encephalomyelopathy) is an inherited neurometabolic disorder that affects the central nervous system. It is named after Archibald Denis Leigh, a British neuropsychiatrist who fi ...
, West syndrome and CAGSSS (cataract
A cataract is a cloudy area in the lens of the eye that leads to a decrease in vision. Cataracts often develop slowly and can affect one or both eyes. Symptoms may include faded colors, blurry or double vision, halos around light, trouble ...
s, growth hormone
Growth hormone (GH) or somatotropin, also known as human growth hormone (hGH or HGH) in its human form, is a peptide hormone that stimulates growth, cell reproduction, and cell regeneration in humans and other animals. It is thus important in h ...
deficiency, sensory neuropathy, sensorineural hearing loss and skeletal dysphasia syndrome).
Prediction servers
ICAARS
B. Pawar, and GPS Raghava (2010) Prediction and classification of aminoacyl tRNA synthetases using PROSITE domains
BMC Genomics 2010, 11:507
*Prokaryotic AAR
database
See also
*TARS (gene)
Threonyl-tRNA synthetase, cytoplasmic is an enzyme that in humans is encoded by the ''TARS'' gene.
Aminoacyl tRNA synthetases catalyze the aminoacylation of tRNA by their cognate amino acid. Because of their central role in linking amino acids w ...
References
External links
* * * {{Authority control EC 6.1 Protein biosynthesis