Anillin on:
[Wikipedia]
[Google]
[Amazon]
Anillin is a conserved protein implicated in cytoskeletal dynamics during cellularization and
cytokinesis
Cytokinesis () is the part of the cell division process during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Cytoplasmic division begins during or after the late stages of nuclear division in mitosis and mei ...
. The ''ANLN'' gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
in humans and the scraps gene in ''Drosophila'' encode Anillin. In 1989, anillin was first isolated in embryos of ''Drosophila melanogaster
''Drosophila melanogaster'' is a species of fly (the taxonomic order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the " vinegar fly" or "pomace fly". Starting with ...
''. It was identified as an F-actin binding protein. Six years later, the anillin gene was cloned from cDNA originating from a Drosophila ovary. Staining with anti-anillin (Antigen 8) antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the ...
showed the anillin localizes to the nucleus during interphase and to the contractile ring during cytokinesis. These observations agree with further research that found anillin in high concentrations near the cleavage furrow coinciding with RhoA, a key regulator of contractile ring formation.
The name of the protein anillin originates from a Spanish word, ''anillo''. ''Anillo'' means ring and shows that the name anillin references the observed enrichment of anillins at the contractile ring during cytokinesis. Anillins are also enriched at other actomyosin rings, most significantly, those at the leading edge of the Drosophila embryo during cellularization. These actomyosin rings invaginate to separate all nuclei for one another in the syncytial blastoderm.
Structure
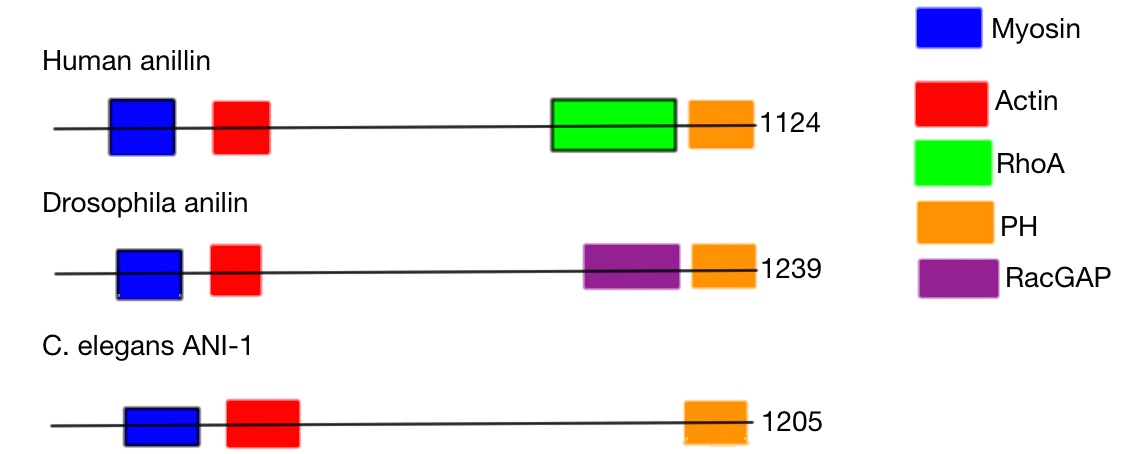
Anillin has a unique multi-domain structure. At the N-terminus, there is an actin- and myosin-binding domain. At the C-terminus, there is aPH domain
In chemistry, pH (), historically denoting "potential of hydrogen" (or "power of hydrogen"), is a scale used to specify the acidity or basicity of an aqueous solution. Acidic solutions (solutions with higher concentrations of ions) are ...
. The PH domain is conserved and essential for anillin functionality. The human anillin cDNA, located on Chr7, encodes a 1,125–amino acid protein with a predicted molecular mass of 124 kD and a pI of 8.1. The mouse anillin gene is located on Chromosome 9.
There are also numerous anillin-like protein homologues found outside of metazoans. In ''Schizosaccharomyces pombe
''Schizosaccharomyces pombe'', also called "fission yeast", is a species of yeast used in traditional brewing and as a model organism in molecular and cell biology. It is a unicellular eukaryote, whose cells are rod-shaped. Cells typically measur ...
'' (fission yeast), there are Mid1p and Mid2p. These two anillin-like proteins do not have any overlap in their functions. Mid1p has been characterized as a key regulator in cytokinesis, responsible for arranging contractile ring
In molecular biology, an actomyosin contractile ring is a prominent structure during cytokinesis.Cheffings TH, Burroughs NJ, Balasubramanian MK. (2016). Actomyosin Ring Formation and Tension Generation in Eukaryotic Cytokinesis. Curr Biol. 26(15): ...
assembly and positioning. Mid2p acts later in cytokinesis to organize septins during septation, or the invagination of inner membranes, outer membranes, and the cell wall that occurs in order to separate daughter cells completely. ''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have b ...
'' (budding yeast) also have two anillin-like proteins, Boi1p and Boi2p. Boi1p and Boi2p localize to the nucleus and contractile ring at the bud neck, respectively. They are essential for cell growth and bud formation.

Function
Anillins are required for the faithfulness of cytokinesis and its F-actin-, myosin-, and septin-binding domains implicate anillin in actomyosin cytoskeletal organization. In agreement with this belief, anillin-mutant cells have disrupted contractile rings. Additionally, it is hypothesized that anillin couples the actomyosin cytoskeleton to microtubules by binding MgcRacGAP/CYK-4/RacGAP50C. Anillins have also been shown to organize the actomyosin cytoskeleton into syncytial structures observed in Drosophila embryos or C. elegans gonads. ANI-1 and ANI-2 (proteins homologous to anillin) are essential for embryonic viability in both organisms. ANI-1 is required for cortical ruffling, pseudocleavage, and all contractile events that occur in embryos prior to mitosis. ANI-1 is also crucial for segregation of polar bodies during meiosis. ANI-2 functions in the maintenance of the structure of the central core of the cytoplasm, therachis
In biology, a rachis (from the grc, ῥάχις [], "backbone, spine") is a main axis or "shaft".
In zoology and microbiology
In vertebrates, ''rachis'' can refer to the series of articulated vertebrae, which encase the spinal cord. In this c ...
, during oogenesis. ANI-2 ensures oocytes do not disconnect prematurely from the rachis, thereby leading to the generation of embryos of varying sizes.
In vitro experiments suggest that anillin drives myosin-independent actin contractility.
Binding Partners
Actin
Anillin specifically binds F-actin, rather than G-actin. Binding of F-actin by anillin only occurs duringcell division
Cell division is the process by which a parent cell divides into two daughter cells. Cell division usually occurs as part of a larger cell cycle in which the cell grows and replicates its chromosome(s) before dividing. In eukaryotes, there ar ...
. Anillin also bundles actin filaments together and drives their relative sliding. This contractile behavior is independent of myosin and ATP and may couple with actin filament disassembly. Amino acids 258-340 are sufficient and necessary for F-actin binding in Drosophila, but amino acids 246-371 are necessary to bundle actin filaments. The ability of anillin to bind to and bundle actin together is conversed through many species. It is hypothesized that by regulating actin bundling, anillin increases the efficiency of actomyosin contractility during cell division. Both anillin and F-actin are found in contractile structures. They are recruited independently to the contractile ring, but F-actin increases the efficiency of anillin targeting. Anillin may also be involved in promoting the polymerization of F-actin by stabilizing formin mDia2 in an active form.
Myosin
Anillin interacts directly with non-muscle myosin II and interacts indirectly with myosin via F-actin. Residues 142-254 (near the N-terminus) are essential for anillin binding myosin in ''Xenopus''. The interaction of anillin and myosin is also dependent on phosphorylation of the myosin light chain. The interaction of myosin and anillin does not seem to serve in recruitment, but rather organization of myosin. In ''Drosophila'', anillin is necessary to organize myosin into rings in the cellularization front. Depletion of anillin in ''Drosophila'' and humans leads to changes in the spatial and temporal stability of myosin during cytokinesis. In C. elegans, ANI-1 organizes myosin into foci during cytokinesis and establishment of polarity, whereas, ANI-2 is a requirement for the maintenance of myosin-rich contractile lining of oogenic gonads.Septins
Septin localization during cytokinesis and cellularization is dependent on its association with anillin. The direct interaction between anillin and septins was first shown by the interaction seen between ''Xenopus'' anillin and a minimal reconstituted heterooligomer of human septins 2, 6, and 7. The ability of anillin to bind to septins is dependent on the C-terminal domain, which contains a terminal PH domain and an upstream sequence known as the “Anillin Homology” (AH) domain.Rho
The AH domain of human anillin is essential for its interaction withRhoA
Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the ''RHOA'' gene. While the effects of RhoA activity are not all well known, it is ...
. Depletion of RhoA halts contractile ring assembly and ingression, whereas, anillin depletion leads to a less severe phenotype when the contractile ring forms and ingresses partially. Depletion of anillin in Drosophila spermatocytes greatly reduces the localization of Rho and F-actin to equatorial regions.
Ect2
Anillin interacts with Ect2, further supporting the idea that anillin stabilizes RhoA localization since Ect2 is an activator of RhoA. Independent of RhoA, the interaction between anillin and Ect2 occurs. This interaction is essential of theGEF
Gef ( ), also referred to as the Talking Mongoose or the Dalby Spook, was the name given to an allegedly talking mongoose which was claimed to inhabit a farmhouse owned by the Irving family. The Irvings' farm was located at Cashen's Gap near ...
activity of Ect2 and requires the AH domain of anillin and the PH domain of Ect2.
Cyk-4
''Drosophila'' anillin interacts with Cyk-4, a central spindle protein, indicating that anillin may have a role in determining the division plane during cytokinesis. In anillin-depleted larval cells, the central spindle does not extend to the cortex. Human anillin-depleted cells show improperly positioned and distorted central spindles.Microtubules
Anillin was first isolated from ''Drosophila'' by harnessing its interactions with both F-actin and microtubules. Furthermore, anillin-rich structures that form after Latrunculin A treatment of ''Drosophila'' cells localize to the plus-ends of microtubules. The interaction between anillin and microtubules suggest that anillin may serve as a signaling factor to relay the position of the mitotic spindle to the cortex to ensure appropriate contractile ring formation during cytokinesis.Regulation
Anillins in metazoans are heavily phosphorylated; however, the kinases responsible for the phosphorylation are unknown at the present time. In humans and ''Drosophila'', anillins are recruited to the equatorial cortex in a RhoA-dependent manner. This recruitment is independent of other cytoskeletal Rho targets such as myosin, F-actin, and Rho-kinase. It has been observed that anillin proteolysis is triggered after mitotic exit by theAnaphase Promoting Complex
Anaphase-promoting complex (also called the cyclosome or APC/C) is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin ...
(APC).
Most anillins can be sequestered to the nucleus during interphase
Interphase is the portion of the cell cycle that is not accompanied by visible changes under the microscope, and includes the G1, S and G2 phases. During interphase, the cell grows (G1), replicates its DNA (S) and prepares for mitosis (G2). A c ...
, but there are exceptions – ''Drosophila'' anilins in the early embryo, C. elegans ANI-1 in early embryos, ''C. elegans'' ANI-2 in oogenic gonads, and Mid2p in fission yeast. These anillins that are not sequestered during interphase suggest that anillins may also regulate cytoskeletal dynamics outside the contractile ring during cytokinesis.
Role in Diseases
Anillin is critical for cell division and therefore development and homeostasis in metazoans. In recent years, the expression levels of anillin have been shown to correlate to the metastatic potential of human tumours. In colorectal cancer, expression levels of anillin are higher in tumours and when anillin was over-expressed in HT29 cells, a classical colorectal cancer cell line, the cells showed faster replication kinetics due to the lengthening of G2/M phase. Increasing the expression of anillin also led to further invasiveness and migration of numerous colorectal cancer cell lines. The hypothesis from such observations is that anillin promotes EMT andcell migration
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing and immune responses all require the orchestrated movement of cells in particular dire ...
through cytoskeletal remodeling, leading to enhanced proliferation, invasion, and mobility of tumour cells.
References
External links
*Further reading
* * * * * * * * * * {{refend Genes Human proteins