Alternative Periodic Table on:
[Wikipedia]
[Google]
[Amazon]
Since Dimitri Mendeleev formulated the
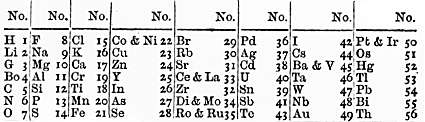

 Short tables have around eight columns. This form became popular following the publication of Mendeleev's eight-column periodic table in 1871.
Also shown in this section is a modernized version of the same table.
Mendeleev and others who discovered chemical periodicity in the 1860s had noticed that when the elements were arranged in order of their atomic weights there was as an approximate repetition of physiochemical properties after every eight elements. Consequently, Mendeleev organized the elements known at that time into a table with eight columns. He used the table to predict the properties of then unknown elements. While his hit rate was less than 50% it was his successes that propelled the widespread acceptance of the idea of a periodic table of the chemical elements. The eight-column style remains popular to this day, most notably in Russia, Mendeleev's country of birth.
An earlier attempt by
Short tables have around eight columns. This form became popular following the publication of Mendeleev's eight-column periodic table in 1871.
Also shown in this section is a modernized version of the same table.
Mendeleev and others who discovered chemical periodicity in the 1860s had noticed that when the elements were arranged in order of their atomic weights there was as an approximate repetition of physiochemical properties after every eight elements. Consequently, Mendeleev organized the elements known at that time into a table with eight columns. He used the table to predict the properties of then unknown elements. While his hit rate was less than 50% it was his successes that propelled the widespread acceptance of the idea of a periodic table of the chemical elements. The eight-column style remains popular to this day, most notably in Russia, Mendeleev's country of birth.
An earlier attempt by

 Long tables have around 32 columns. Early examples are given by Bassett (1892), with 37 columns arranged albeit vertically rather than horizontally; Gooch & Walker (1905),
with 25 columns; and by Werner (1905), with 33 columns.
In the first image in this section, of a so-called left step table:
* Groups 1 and 2 (the
Long tables have around 32 columns. Early examples are given by Bassett (1892), with 37 columns arranged albeit vertically rather than horizontally; Gooch & Walker (1905),
with 25 columns; and by Werner (1905), with 33 columns.
In the first image in this section, of a so-called left step table:
* Groups 1 and 2 (the
 Encompassing circular,
Encompassing circular,
 Unclassified periodic tables defy easy classification:
Unclassified periodic tables defy easy classification:
File:ADOMAH periodic table - electron orbitals (polyatomic).svg, ADOMAH (long)
File:The chemical elements and their periodic relationships.svg, Curled ribbon (continuous)
File:Discoid table.jpg, Discoid (circular)
File:Mendeleev flower.jpg, Four loops (continuous)
File:Partially disordered PT.png, Partially disordered (unclassified)
File:Hackh's PT (1918).png, Short (9/11 columns)
File:Notes to Hackh's PT (1918).png, Short (9/11 columns) notes
File:Periodic table (spiral format).SVG, Spiral
File:Periodic ziggurat part 1.png, Ziggurat (unclassified)
File:Periodic ziggurat part 2.png, Ziggurat notes
File:4DPeriodicTable.png, 4D Stowe-Scerri
Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements
Doctoral Dissertations (1385), University of Massachusetts, Amherst *Semenov NN 1969, 100 лет периодического закона химических элементов. 1869-1969 (100 years of the periodic law of chemical elements. 1869-1969), in Russian, Nauka, Moscow *Venable FP 1896, ''The Development of the Periodic Law,'' Chemical Publishing Co., Easton, PA
The INTERNET Database of Periodic Tables
{{DEFAULTSORT:Types of Periodic Tables Periodic table
periodic law
Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. They were discovered by the Russian chemist Dmitri Mendeleev in the year 1863. Major periodic trends include atom ...
in 1871, and published an associated periodic table of chemical elements, authors have experimented with varying types of periodic tables including for teaching, aesthetic or philosophical purposes.
Earlier, in 1869, Mendeleev had mentioned different layouts including short, medium, and even cubic forms. It appeared to him that the latter (three-dimensional) form would be the most natural approach but that "attempts at such a construction have not led to any real results". On spiral periodic tables, "Mendeleev...steadfastly refused to depict the system as uch
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab, Pakistan, Punjab province. Uch may have been founded as Alexandria on the Indus, a town ...
..His objection was that he could not express this function mathematically."
Typology
In 1934, George Quam, a chemistry professor at Long Island University, New York, and Mary Quam, a librarian at the New York Public Library compiled and published a bibliography of 133 periodic tables using a five-fold typology: I. short; II. long (including triangular); III. spiral; IV. helical, and V. miscellaneous. In 1952,Moeller
Moeller and Möller are closely related surnames of German origin.
People bearing one of them include the following:
People
* Adolph Moeller, American politician
* Alfred Alphonse Moeller (1889–1971), governor of Orientale Province in the Bel ...
expressed disdain as to the many types of periodic table:
In 1954, Tomkeieff referred to the three principle types of periodic table as helical, rectilinear, and spiral. He added that, "unfortunately there also a number of freaks".
In 1974 Edward Mazurs, a professor of chemistry, published a survey and analysis of about seven hundred periodic tables that had been published in the preceding one hundred years; he recognized short, medium, long, helical, spiral, series tables, and tables not classified.
In 1999 Mark Leach, a chemist, inaugurated the INTERNET database of Periodic Tables. It has over 1200 entries as of May 2023. While the database is a chronological compilation, specific types of periodic tables that can be searched for are spiral and helical; 3-dimensional; and miscellaneous.
For convenience, periodic tables may be typified as either: 1. short; 2. triangular; 3. medium; 4. long; 5. continuous (circular, spiral, lemniscate, or helical); 6. folding; or 7. spatial. Tables that defy easy classification are counted as type 8. unclassified.
Short


 Short tables have around eight columns. This form became popular following the publication of Mendeleev's eight-column periodic table in 1871.
Also shown in this section is a modernized version of the same table.
Mendeleev and others who discovered chemical periodicity in the 1860s had noticed that when the elements were arranged in order of their atomic weights there was as an approximate repetition of physiochemical properties after every eight elements. Consequently, Mendeleev organized the elements known at that time into a table with eight columns. He used the table to predict the properties of then unknown elements. While his hit rate was less than 50% it was his successes that propelled the widespread acceptance of the idea of a periodic table of the chemical elements. The eight-column style remains popular to this day, most notably in Russia, Mendeleev's country of birth.
An earlier attempt by
Short tables have around eight columns. This form became popular following the publication of Mendeleev's eight-column periodic table in 1871.
Also shown in this section is a modernized version of the same table.
Mendeleev and others who discovered chemical periodicity in the 1860s had noticed that when the elements were arranged in order of their atomic weights there was as an approximate repetition of physiochemical properties after every eight elements. Consequently, Mendeleev organized the elements known at that time into a table with eight columns. He used the table to predict the properties of then unknown elements. While his hit rate was less than 50% it was his successes that propelled the widespread acceptance of the idea of a periodic table of the chemical elements. The eight-column style remains popular to this day, most notably in Russia, Mendeleev's country of birth.
An earlier attempt by Newlands
Newlands may refer to:
Places Australia
* Newlands, Queensland, a locality in the Whitsunday Region
New Zealand
* Newlands, Wellington, a suburb of Wellington
South Africa
* Newlands, Cape Town, a suburb of Cape Town
* Newlands, Johannesbur ...
, an English chemist, to present the nub of the same idea to the London Chemical Society
London is the capital and largest city of England and the United Kingdom, with a population of just under 9 million. It stands on the River Thames in south-east England at the head of a estuary down to the North Sea, and has been a major se ...
, in 1866, was unsuccessful; members were less than receptive to theoretical ideas, as was the British tendency at the time. He referred to his idea as the Law of Octaves
The periodic table is an arrangement of the chemical elements, structured by their atomic number, electron configuration and recurring chemical properties. In the basic form, elements are presented in order of increasing atomic number, in the r ...
, at one point drawing an analogy with an eight-key musical scale.
John Gladstone, a fellow chemist, objected on the basis that Newland's table presumed no elements remained to be discovered. "The last few years had brought forth thallium, indium, caesium, and rubidium, and now the finding of one more would throw out the whole system." He believed there was as close an analogy between the metals named in the last vertical column as in any of the elements standing on the same horizontal line.
Fellow English chemist Carey Foster
George Carey Foster FRS (October 1835 – 9 February 1919) was a chemist and physicist, born at Sabden in Lancashire. He was Professor of Physics at University College London, and served as the first Principal (salaried head of the College) fr ...
humorously inquired of Newlands whether he had ever examined the elements according to the order of their initial letters. Foster believed that any arrangement would present occasional coincidences, but he condemned one which placed so far apart manganese and chromium, or iron from nickel and cobalt.
The advantages of the short form of periodic table are its compact size and that it shows the relationships between main group elements and transition metal groups
Its disadvantages are that it appears to group dissimilar elements, such as chorine and manganese, together; the separation of metals and nonmetals is hard to discern; there are "inconsistencies in the grouping together of elements giving colorless, diamagnetic ions with elements giving colored, paramagnetic ions; and lack of reasonable positions for hydrogen, the lanthanide elements, and the actinide elements."
Some other notable short periodic tables include:
Triangular
Triangular tables have column widths of 2-8-18-32 or thereabouts. An early example, appearing in 1882, was provided by Bayley. Through the use of connecting lines, such tables make it easier to indicate analogous properties among the elements. In some ways they represent a form intermediate between the short and medium tables, since the average width of the fully mature version (with widths of 2+8+18+32 = 60) is 15 columns. An early drawback of this form was to make predictions for missing elements based on considerations of symmetry. For example, Bayely considered therare earth metals
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silve ...
to be indirect analogues of other elements such as, for example, zirconium and niobium, a presumption which turned out to be largely unfounded.
Advantages of this form are its aesthetic appeal, and relatively compact size; disadvantages are its width, the fact that it is harder to draw, and interpreting certain periodic trends or relationships may be more challenging compared to the traditional rectangular format.
Some other notable triangular periodic tables include:
Medium
Medium tables have around 18 columns. The popularity of this form is thought to be a result of it having a good balance of features in terms of ease of construction and size, and its depiction of atomic order and periodic trends. Deming's version of a medium table, which appeared in the first edition of his 1923 textbook "General Chemistry: An Elementary Survey Emphasizing Industrial Applications of Fundamental Principles", has been credited with popularizing the 18-column form. LeRoy referred to Deming's table, "this...being better known as the 'eighteen columns'-form" as representing "a very marked improvement over the original Mendeleef type as far as presentation to beginning classes is concerned." Merck and Company prepared a handout form of Deming's table, in 1928, which was widely circulated in American schools. By the 1930s his table was appearing in handbooks and encyclopedias of chemistry. It was also distributed for many years by the Sargent-Welch Scientific Company. The advantages of the medium form are that it correlates the positions of the elements with their electronic structures, accommodates the vertical, horizontal and diagonal trends that characterise the elements;, and separates the metals and nonmetals; its disadvantages are that it obscures the relationships between main group elements and transition metals. Some other notable medium tables include:Long

s-block
A block of the periodic table is a set of elements unified by the atomic orbitals their valence electrons or vacancies lie in. The term appears to have been first used by Charles Janet. Each block is named after its characteristic orbital: s-blo ...
) have been moved to the right side of the table.
* The s-block is shifted up one row, thus all elements not in the s-block are now one row lower than in the standard table. For example, most of the fourth row in the standard table is the fifth row in this table.
* Helium is placed in group 2 (not in group 18).
The elements remain positioned in order of atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of every ...
(''Z'').
The left step table was developed by Charles Janet
Charles Janet (; 15 June 1849 – 7 February 1932) was a French engineer, company director, inventor and biologist. He is also known for his innovative ''left-step'' presentation of the periodic table of chemical elements.
Life and work
Janet gr ...
, in 1928, originally for aesthetic purposes. That being said it shows a reasonable correspondence with the Madelung energy ordering rule this being a notional sequence in which the electron shells of the neutral atoms in their ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
s are filled.
A more conventional long form of periodic table is included for comparison.
The advantage of the long form is that shows where the lanthanides and actinides fit into the periodic table; its disadvantage is its width.
Some other notable long tables include:
Continuous
spiral
In mathematics, a spiral is a curve which emanates from a point, moving farther away as it revolves around the point.
Helices
Two major definitions of "spiral" in the American Heritage Dictionary are:lemniscate
In algebraic geometry, a lemniscate is any of several figure-eight or -shaped curves. The word comes from the Latin "''lēmniscātus''" meaning "decorated with ribbons", from the Greek λημνίσκος meaning "ribbons",. or which alternativel ...
, or helical
Helical may refer to:
* Helix, the mathematical concept for the shape
* Helical engine, a proposed spacecraft propulsion drive
* Helical spring, a coilspring
* Helical plc, a British property company, once a maker of steel bar stock
* Helicoil
A t ...
tables.
The Crookes' lemniscate periodic table shown in this section has the following elements falling under one another:
The collocation of manganese with iron, nickel and cobalt is later seen in the modernised version of von Bichowsky's table of 1918, in the unclassified section of this article.
The French geologist, Alexandre-Émile Béguyer de Chancourtois was the first person to make use of atomic weights to produce a classification of periodicity. He drew the elements as a continuous spiral around a metal cylinder divided into 16 parts. The atomic weight of oxygen was taken as 16 and was used as the standard against which all the other elements were compared. Tellurium was situated at the centre, prompting ''vis tellurique'', or ''telluric screw''.
The advantage of this form is that it emphasizes, to a greater or lesser degree, that the elements form a continuous sequence; that said, continuous tables are harder to construct, read and memorize than the traditional rectangular form of periodic table.
Some other notable forms of continuous periodic tables include:
Folding
Such tables, which incorporate a folding mechanism, are relatively uncommon: The advantages of such tables are their novelty and that they can depict relationships that ordinarily require spatial periodic tables, yet retain the portability and convenience of two-dimensional tables. A disadvantage is that they require marginally more effort to construct.Spatial
Spatial tables pass through three or more dimensions (helical tables are instead classed as continuous tables). Such tables are relatively niche and not as commonly used as traditional tables. While they offer unique advantages, their complexity and customization requirements make them more suitable for specialized research, advanced education, or specific areas of study where a deeper understanding of multidimensional relationships is desired. Advantages of periodic tables of three or more dimensions include: *Enhanced visualization. Such tables provide a unique and enhanced visualization of the elements and their properties. By incorporating additional dimensions, such as depth or multiple axes, these tables offer a more comprehensive representation of the periodic trends and relationships among the elements. They can provide a richer understanding of complex patterns and interactions. *Inclusion of extra properties: Traditional periodic tables typically focus on a few key properties, such as atomic number and atomic weight. However, periodic tables of three or more dimensions have the potential to include additional properties, such as electronegativity, ionization energy, electron affinity, or physical properties like boiling point or melting point. This expanded information can offer a more complete picture of the elements and their characteristics. *Exploration of higher-level trends: Such tables can facilitate the exploration of higher-level trends and relationships that may not be apparent in traditional two-dimensional tables. They allow for the visualization of complex patterns that emerge when multiple properties or variables are considered simultaneously. This can aid in uncovering hidden connections and correlations among the elements. *Flexibility and customization: Periodic tables of three or more dimensions offer flexibility in terms of their design and customization. Researchers, educators, or scientists can adapt the dimensions and properties represented based on their specific needs and objectives. This adaptability allows for tailoring the table to focus on specific areas of interest or research. Disadvantages are: *Complexity: As the number of dimensions increases, the complexity of interpreting and understanding the table also increases. It can become more challenging for individuals to grasp and visualize the relationships between elements, especially when multiple properties are incorporated. The intricate nature of these tables may require additional effort and familiarity to navigate and interpret effectively. *Difficulty in representation: Depicting periodic trends and relationships in three or more dimensions can be technically challenging. Designing and visualizing the table in a clear and coherent manner may require specialized software or tools. The complexity of these tables can make them less accessible for individuals who are not familiar with the specific representation or visualization techniques used. *Information overload: The inclusion of multiple dimensions and properties can lead to information overload, especially if the table is not designed in a user-friendly and organized manner. It becomes crucial to effectively organize and present the data to avoid overwhelming users with excessive details. Striking a balance between comprehensive information and clarity can be a significant challenge. *Lack of standardization: Periodic tables of three or more dimensions are not as standardized or widely recognized as traditional two-dimensional tables. This lack of standardization can create confusion and inconsistency across different representations. It can also make it more difficult to compare and communicate information between different periodic table formats. Some other notable spatial periodic tables include:Unclassified
 Unclassified periodic tables defy easy classification:
Unclassified periodic tables defy easy classification:
Gallery
Notes
References
Further reading
*Blokh MA 1934, Iubileinomu mendeleevskomu s'ezdu v oznamenovanie 100-letnei godovshchinyso dnia rozhdeniia D. I. Mendeleeva (Anniversary Mendeleev Congress in commemoration of the 100th anniversary of the birth of D. I. Mendeleev), in Russian, Akad. Nauk SSSR, Leningrad * Mazurs EG 1974, ''Graphic Representations of the Periodic System During One Hundred Years,'' University of Alabama Press, Alabama, ISBN 978-0-8173-3200-6 *Quam GN & Quam MB 1934, Types of graphic classifications of the elements I. Introduction and short tables, ''Journal of Chemical Education,'' vol. 11, no. 1, pp. 27–32, *id., Types of graphic classifications of the elements II. Long charts, ''Journal of Chemical Education,'' vol. 11, no. 4, pp. 217–223, *id., Types of graphic classifications of the elements III. Spiral, helical, and miscellaneous charts, ''Journal of Chemical Education,'' vol. 11, no. 5, pp. 288–297, *Robinson A 2018Creating a Symbol of Science: The Development of a Standard Periodic Table of the Elements
Doctoral Dissertations (1385), University of Massachusetts, Amherst *Semenov NN 1969, 100 лет периодического закона химических элементов. 1869-1969 (100 years of the periodic law of chemical elements. 1869-1969), in Russian, Nauka, Moscow *Venable FP 1896, ''The Development of the Periodic Law,'' Chemical Publishing Co., Easton, PA
External links
*Leach M 1999 to dateThe INTERNET Database of Periodic Tables
{{DEFAULTSORT:Types of Periodic Tables Periodic table