alkyl ketene dimer on:
[Wikipedia]
[Google]
[Amazon]
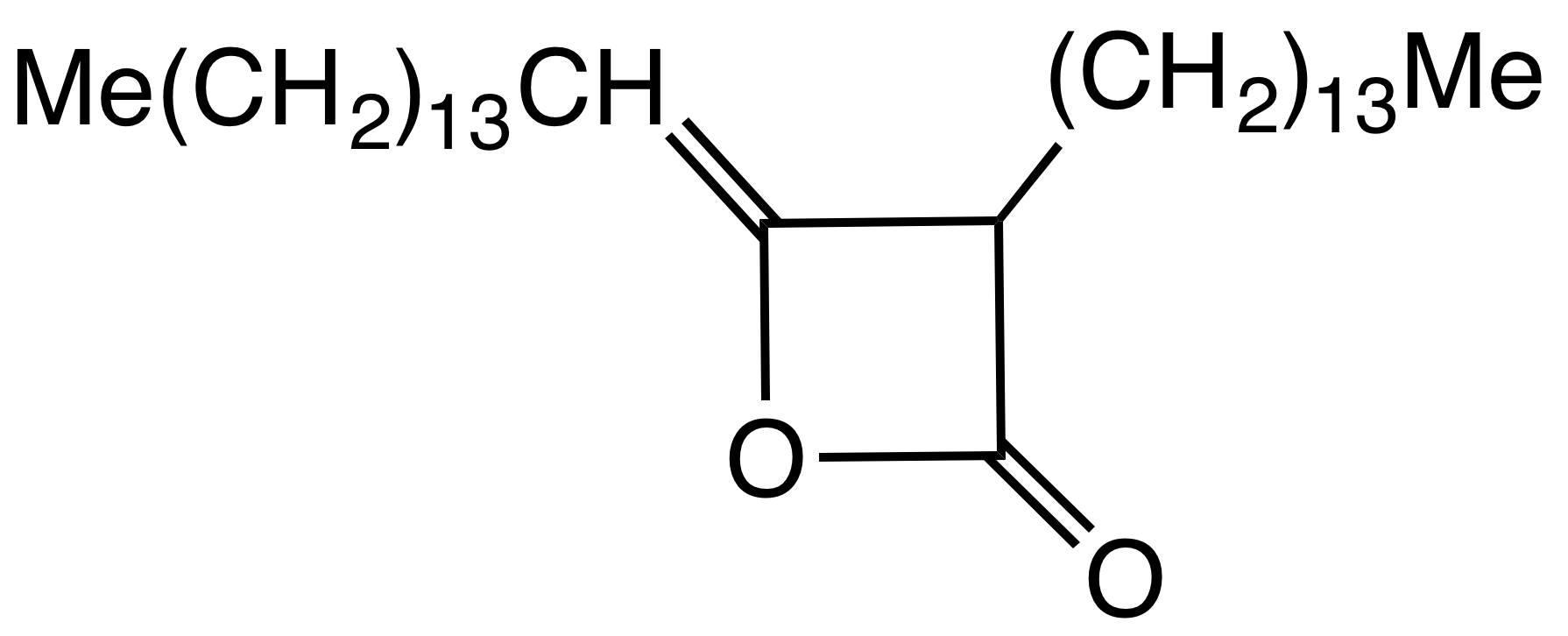 Alkyl ketene dimers (AKDs) are a family of
Alkyl ketene dimers (AKDs) are a family of
 The molecular weight determined by the early researchers did indicate a multiple of the group R1R2CH=C=O. Therefore, a so-called pyronone (a diketone structure with a cyclobutane ring) was proposed as reaction product, e. g. from the reaction of
The molecular weight determined by the early researchers did indicate a multiple of the group R1R2CH=C=O. Therefore, a so-called pyronone (a diketone structure with a cyclobutane ring) was proposed as reaction product, e. g. from the reaction of  The primary reaction products of carboxylic acid chlorides with hydrogen atoms in α-position and tertiary amines were identified by
The primary reaction products of carboxylic acid chlorides with hydrogen atoms in α-position and tertiary amines were identified by  The 2,2,4,4,4-tetramethylcyclobutanedione can easily be isomerized to dimethyl ketene dimer (4-isopropylidene-3,3-dimethyl-oxetan-2-one).
The 2,2,4,4,4-tetramethylcyclobutanedione can easily be isomerized to dimethyl ketene dimer (4-isopropylidene-3,3-dimethyl-oxetan-2-one).
 The synthesis and characterization of hexadecyl ketene dimer, a key substance for alkylated ketene dimers used in the paper industry, was first described in a patent in 1945 and in a publication in 1947.
A quantum-chemical study rejected the formation of a cyclobutanedione during the dimerization of ''n''-alkylketene R-CH=C=O in favour of the formation of the thermodynamically more stable oxetan-2-one structure.
The synthesis and characterization of hexadecyl ketene dimer, a key substance for alkylated ketene dimers used in the paper industry, was first described in a patent in 1945 and in a publication in 1947.
A quantum-chemical study rejected the formation of a cyclobutanedione during the dimerization of ''n''-alkylketene R-CH=C=O in favour of the formation of the thermodynamically more stable oxetan-2-one structure.
 The use of other solvents, such as
The use of other solvents, such as
 Industrially applied AKDs are derived from fatty acids with chain lengths between C14 (
Industrially applied AKDs are derived from fatty acids with chain lengths between C14 (
 The molecular structure (i.e. molar mass and cross-linking degree), the molar charge density of cationic groups, the exact dosage of the cationic polymer as a dispersion stabilizer and retention aid as well as keeping the other process parameters such as temperature, pH and residence times is crucial.
After removal of excess water - also to avoid hydrolysis of the AKD to the beta-keto acid and subsequent
The molecular structure (i.e. molar mass and cross-linking degree), the molar charge density of cationic groups, the exact dosage of the cationic polymer as a dispersion stabilizer and retention aid as well as keeping the other process parameters such as temperature, pH and residence times is crucial.
After removal of excess water - also to avoid hydrolysis of the AKD to the beta-keto acid and subsequent  follows the cracking of the stabilized AKD particles on the base paper mass, the melting of the solid AKD wax (at approx. 90 °C), the spreading of the liquid AKD wax by
follows the cracking of the stabilized AKD particles on the base paper mass, the melting of the solid AKD wax (at approx. 90 °C), the spreading of the liquid AKD wax by
 Alkyl ketene dimers (AKDs) are a family of
Alkyl ketene dimers (AKDs) are a family of organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ...
s based on the 4-membered ring system of oxetan-2-one, which is also the central structural element of propiolactone Propiolactone may refer to either of two isomeric chemical compounds:
* Alpha-Propiolactone (α-Propiolactone)
* Beta-Propiolactone
β-Propiolactone is an organic compound of the lactone family, with a four-membered ring. It is a colorless liq ...
and diketene
Diketene is an organic compound with the molecular formula , and which is sometimes written as . It is formed by dimerization of ketene, . Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorles ...
. Attached to the oxetane ring of technically relevant alkyl ketene dimers there is a C12 – C16 alkyl group
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloalk ...
in the 3-position and a C13 – C17 alkylidene group in the 4-position.
The main application of alkylated ketene dimers is in the sizing
Sizing or size is a substance that is applied to, or incorporated into, other materials—especially papers and textiles—to act as a protective filler or glaze. Sizing is used in papermaking and textile manufacturing to change the absorption ...
of paper
Paper is a thin sheet material produced by mechanically or chemically processing cellulose fibres derived from wood, rags, grasses or other vegetable sources in water, draining the water through fine mesh leaving the fibre evenly distributed ...
and cardboard
Cardboard is a generic term for heavy paper-based products. The construction can range from a thick paper known as paperboard to corrugated fiberboard which is made of multiple plies of material. Natural cardboards can range from grey to light b ...
, as well as in the hydrophobation of cellulose fiber
Cellulose fibers () are fibers made with ethers or esters of cellulose, which can be obtained from the bark, wood or leaves of plants, or from other plant-based material. In addition to cellulose, the fibers may also contain hemicellulose and l ...
s. The products thus modified are distinguished by higher mechanical strengths and less penetration of water, ink
Ink is a gel, sol, or solution that contains at least one colorant, such as a dye or pigment, and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, reed pen, or quill. Thi ...
s or printing ink
Ink is a gel, sol, or solution that contains at least one colorant, such as a dye or pigment, and is used to color a surface to produce an image, text, or design. Ink is used for drawing or writing with a pen, brush, reed pen, or quill. Thicke ...
s.
AKD's feature hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
alkyl groups extending from a beta-propiolactone
β-Propiolactone is an organic compound of the lactone family, with a four-membered ring. It is a colorless liquid with a slightly sweet odor, highly soluble in water and miscible with ethanol, acetone, diethyl ether and chloroform.''Merck Index ...
ring. A specific example is derived from the dimerization of the ketene of stearic acid
Stearic acid ( , ) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a waxy solid and its chemical formula is C17H35CO2H. Its name comes from the Greek word στέαρ "''stéar''", which means tallow. ...
. This ketene is generated by pyrolysis of stearoyl chloride. AKD's react with the hydroxyl groups on the cellulose via esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
reaction. The esterification is competitive with hydrolysis of the AKD. Prior to the development of AKD's, hydrophobicity was imparted by incorporating rosin
Rosin (), also called colophony or Greek pitch ( la, links=no, pix graeca), is a solid form of resin obtained from pines and some other plants, mostly conifers, produced by heating fresh liquid resin to vaporize the volatile liquid terpene comp ...
into the paper.
Related to AKDs, is alkenylsuccinic anhydride (ASA). As for AKDs, ASA reacts with hydroxy groups of the cellulose to form an ester, anchoring the hydrophobic group to the surface. ASA is prepared by the ene reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile ...
of unsaturated hydrocarbons with maleic anhydride
Maleic anhydride is an organic compound with the formula C2H2(CO)2O. It is the acid anhydride of maleic acid. It is a colorless or white solid with an acrid odor. It is produced industrially on a large scale for applications in coatings and poly ...
.
History
As early as 1901, Edgar Wedekind published the synthesis of alkyl ketene dimers by the reaction of carboxylic acid chlorides withtertiary amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such ...
, the reaction products for polymers.
isobutyryl chloride
Isobutyryl chloride (2-methylpropanoyl chloride) is the simplest branched-chain acyl chloride. It is found at room temperature as a corrosive, colorless liquid.
References
Acyl chlorides
Reagents for organic chemistry
{{Organic-compoun ...
and triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA ...
.
Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
and Norman Thomas Mortimer Wilsmore as highly reactive ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound etheno ...
s (ethenones) which form 2-oxetanones with an alkylidene group when dimerizing in a +2photocycloadditions. This gradually brought clarity about the constitution of alkylated ketene dimers.
The clarification of the constitution
A constitution is the aggregate of fundamental principles or established precedents that constitute the legal basis of a polity, organisation or other type of Legal entity, entity and commonly determine how that entity is to be governed.
When ...
was complicated by different dimerization products of the ketenes. For example, the simple ketene (H2C=C=O) dimerizes to diketene (4-methylen-oxetan-2-one), while substituted ketenes, such as dimethyl ketene (Me2C=C=O, formed from isobutyryl chloride with triethylamine) dimerize in a head-to-tail addition to 2,2,4,4-tetramethylcyclobutanedione.
Preparation
The industrial synthesis of alkylated ketenedimers (at that time still called ketoethenones) was patented in 1945 from long-chain carboxylic acid chlorides in inert solvents (such asdiethyl ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liq ...
or benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, ...
) with triethylamine as tertiary amine under anhydrous conditions. After filtration of the insoluble triethylamine hydrochloride and evaporation of the solvent, long-chain alkyl chain dimers are obtained in yields of more than 90%.
carboxylic acid ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fa ...
s or ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bo ...
s for easier separation of trialkylamine hydrochlorides or other amines, such as ''N,N,N',N-tetramethyl-hexane-1,6-diamine does not provide any significant advantages.
Also processes without the solvent use are described, in which the resulting amine hydrochloride is either filtered off or extracted with diluted aqueous acids.
A continuous process in which long-chain carboxylic acid chloride and tertiary amine (e. g. dimethyl isopropylamine, dimethylcyclohexylamine or triethylamine) is supplied separately without solvents to a tube reactor, kneader or preferably a twin-screw extruder or planetary roller extruder and reacted at temperatures between 90 and 110 °C, delivers lactone contents of over 90% at short reaction times. Processing is carried out by phase separation or acidic extraction.
Use
Alkylated ketene dimers as paper sizing agents
The problems with the acidic (aluminum sulfate-mediated) mass sizing of paper with alkaline-digested colophony resins introduced since the early 19th century led besides the use of alkaline flocculants (such aschalk
Chalk is a soft, white, porous, sedimentary carbonate rock. It is a form of limestone composed of the mineral calcite and originally formed deep under the sea by the compression of microscopic plankton that had settled to the sea floor. Chalk ...
or calcium carbonate as the alkali reserve) to the search for alternative materials for sizing in a neutral or alkaline environment. In addition to the significantly more reactive alkenylsuccinic anhydride
Alkenyl succinic anhydrides (ASA) are modified five-membered succinic anhydrides bearing a branched iso-alkenyl chain (C14 to C22). They are colorless, and usually viscous liquids. They are widely used, especially in surface sizing of paper, paper ...
s (which do also hydrolyze rapidly in the presence of water) alkylated ketene dimers have begun to be preferred surface and mass sizes in the paper industry from the 1960s onwards, beginning in the 1950s.
myristic acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nutm ...
) to C22 (behenic acid
Behenic acid (also docosanoic acid) is a carboxylic acid, the saturated fatty acid with formula C21H43COOH. In appearance, it consists of white solid although impure samples appear yellowish.
Sources
At 9%, it is a major component of ben oil (or ...
); palmityl (C16) diketene and stearyl (C18) ketene and mixtures thereof are preferably used, as well as fatty acid mixtures from the hydrolysis of animal and vegetable fats. Because of the chain length of the original fatty acids, AKD are waxy solids with melting points between 42 and about 70 °C. Mixtures of alkylated ketene dimers and water are dispersions at temperatures below 40 °C or emulsion
An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althoug ...
s at temperatures above 45 °C. Liquid AKD are not widely used, they are based on unsaturated fatty acids like oleic acid
Oleic acid is a fatty acid that occurs naturally in various animal and vegetable fats and oils. It is an odorless, colorless oil, although commercial samples may be yellowish. In chemical terms, oleic acid is classified as a monounsaturated omega ...
or branched fatty acids, like isostearic acid.
Aqueous alkyldiketene dispersions generally contain 10-20 wt% of AKD, as well as active protective colloids (particularly polycation
Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are t ...
s such as cationic starch, copolymers of ''N''-vinylpyrrolidone and quaternized ''N''-vinylimidazole, acylated polyethyleneimines or cationic high molecular weight polyacrylamides with an average molar mass up to 7 million g/mol) and other stabilizers (usually anionic surfactants, for example ligninsulfonates or condensation products of naphthalenesulfonic acid sodium salt and formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
). Such stabilized AKD dispersions are active and stable at room temperature for up to three months and also tolerate the addition of different fillers for paper or cardboard (e.g. kaolin
Kaolinite ( ) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is an important industrial mineral. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral ...
, chalk, talc, titanium dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or CI 77891. It is a white solid that is insolubl ...
, calcium sulfate
Calcium sulfate (or calcium sulphate) is the inorganic compound with the formula CaSO4 and related hydrates. In the form of γ-anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Pari ...
, aluminum oxide, etc.) from 5 to 25%. The amounts of alkyl ketene dimers used for the sizing of paper and paper products are preferably in the range from 0.15 to 0.8 wt%, sometimes from 0.05 to 0.2 wt%, based on the dry paper stock.
Paper sizing with alkylated ketene dimers
For paper sizing with AKD, a three-step process was proposed which, despite controversial discussions in the 1990s, seems to describe the processes that are taking place best and explains the results achieved. Decisive criteria for the quality of the hydrophobicity of papers are # the retention of the AKD particles on the wet paper mass on the paper screen # the spreading of the AKD particles on the surface and the penetration in the paper mass # the chemical reaction of thehydroxyl groups
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
of the cellulose (esterification
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
) with the alkylated ketene dimers to form beta-ketocarboxylic esters.
decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is t ...
to the ketone -
surface diffusion
Surface diffusion is a general process involving the motion of adatoms, molecules, and atomic clusters ( adparticles) at solid material surfaces.Oura, Lifshits, Saranin, Zotov, and Katayama 2003, p. 325 The process can generally be thought of in t ...
on the cellulose fibers and the formation of closed hydrophobic layers. The thickness of the hydrophobic layers depends on the AKD concentration in the dispersion.
Ad 3. The hydrophobation of cellulose fibers with alkylated ketene dimers takes place most effectively in neutral or preferably weakly alkaline media (pH 7.5-9.0). The reaction temperature is generally 90-110 °C, with approximately 40% of the AKD used reacting with the cellulose. After the reaction contact angle
The contact angle is the angle, conventionally measured through the liquid, where a liquid–vapor interface meets a solid surface. It quantifies the wettability of a solid surface by a liquid via the Young equation. A given system of solid, liq ...
s of >100° are measured, indicating the hydrophobic character of the AKD-modified model surfaces. The esterification of hydroxyl groups of cellulose fibers was also demonstrated by comparison reactions with 14C-labeled AKD.
The sizing with AKD is suitable for the permanent hydrophobation of newspaper, printing and writing paper and cardboard used as a container for liquids (including foodstuffs such as milk), as well as for the improvement of shape stability and runnability.
Literature
* *{{citation, first=D., last=Johnson, editor-surname1=I. Thorn, C.O. Au, title=Applications of Wet-End Paper Chemistry, 2nd Edition, publisher=Springer Netherlands, pages=73–112, isbn=978-1-4020-6037-3, date=2009References