2,3-sigmatropic Rearrangement on:
[Wikipedia]
[Google]
[Amazon]
2,3-Sigmatropic rearrangements are a type of  A ,3rearrangement may result in carbon-carbon bond formation. It can also be used as a ring-expansion reaction.''Ring expansion by 2,3-sigmatropic shifts of unstabilized sulfonium ylides. Synthesis of eight- to ten-membered thiacycloalk-4-enes'' V. Cere, C. Paolucci, S. Pollicino, E. Sandri, and A. Fava The Journal of Organic Chemistry 1978 43 (25), 4826-4831
A ,3rearrangement may result in carbon-carbon bond formation. It can also be used as a ring-expansion reaction.''Ring expansion by 2,3-sigmatropic shifts of unstabilized sulfonium ylides. Synthesis of eight- to ten-membered thiacycloalk-4-enes'' V. Cere, C. Paolucci, S. Pollicino, E. Sandri, and A. Fava The Journal of Organic Chemistry 1978 43 (25), 4826-4831
sigmatropic rearrangement
In organic chemistry, a sigmatropic reaction () is a pericyclic reaction wherein the net result is one sigma bond (σ-bond) is changed to another σ-bond in an intramolecular reaction. In this type of rearrangement reaction, a substituent moves f ...
s and can be classified into two types. Rearrangements of allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
ic sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s, amine oxide
An oxide () is a chemical compound containing at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion (anion bearing a net charge of −2) of oxygen, an O2− ion with oxygen in the oxidation st ...
s, selenoxide
Organoselenium chemistry is the science exploring the properties and reactivity of organoselenium compounds, chemical compounds containing carbon-to-selenium chemical bonds. Selenium belongs with oxygen and sulfur to the group 16 elements or chalco ...
s are neutral
Neutral or neutrality may refer to:
Mathematics and natural science Biology
* Neutral organisms, in ecology, those that obey the unified neutral theory of biodiversity
Chemistry and physics
* Neutralization (chemistry), a chemical reaction in ...
. Rearrangements of carbanion
In organic chemistry, a carbanion is an anion with a lone pair attached to a tervalent carbon atom. This gives the carbon atom a negative charge.
Formally, a carbanion is the conjugate base of a carbon acid:
:
where B stands for the base (chemist ...
s of allyl ether
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R� ...
s are anionic
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
. The general scheme for this kind of rearrangement is:
Atom Y may be sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
, selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
, or nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
. If Y is nitrogen, the reaction is referred to as the Sommelet–Hauser rearrangement if a quaternary ammonium salt is involved or the aza-Wittig reaction if an alpha-metalated tertiary amine is involved; if Y is oxygen, then it is called a 2,3-Wittig rearrangement (not to be confused with the well-known Wittig reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most o ...
, which involves a phosphonium ylide
An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both ...
). If Y is sulfur, the product can be treated with a thiophil to generate an allylic alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
in what is known as the Mislow–Evans rearrangement The Mislow–Evans rearrangement is a name reaction in organic chemistry. It is named after Kurt Mislow who reported the prototypical reaction in 1966, and David A. Evans who published further developments. The reaction allows the formation of allyl ...
.
Stereoselectivity
2,3-sigmatropic rearrangements can offer highstereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
. At the newly formed double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
there is a strong preference for formation of the E-alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
or trans isomer
Trans- is a Latin prefix meaning "across", "beyond", or "on the other side of".
Used alone, trans may refer to:
Sociology
* Trans, a sociological term which may refer to:
** Transgender, people who identify themselves with a gender that dif ...
product. The stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
of the newly formed C-C bond is harder to predict. It can be inferred from the five-membered ring transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
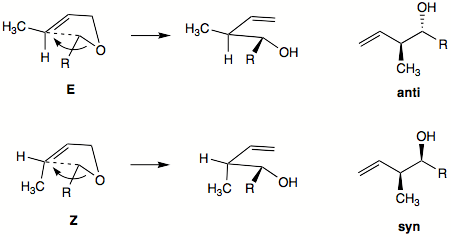
. Generally, the E-alkene will favor the formation of anti product, while Z-alkene will favor formation of syn product.

Diastereoselectivity
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have d ...
can be high for Z-alkene with alkynyl, alkenyl, or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
anion-stabilizing group. Diastereoselectivity is usually lower with E-alkenes. Hydrocarbon groups will prefer exo
Exo (; stylized in all caps) is a South Korean-Chinese boy band based in Seoul formed by SM Entertainment in 2011 and debuted in 2012. The group consists of nine members: Xiumin, Suho, Lay Zhang, Lay, Baekhyun, Chen (singer), Chen, Chanyeol, ...
orientation in the envelope-like transition state. Anion-stabilizing group will prefer the endo orientation in transition state.
References
{{Reflist Rearrangement reactions Reaction mechanisms