|
Sigmatropic Rearrangement
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compounding of the long-established sigma designation from single carbon–carbon bonds and the Greek word ''tropos'', meaning turn. In this type of rearrangement reaction, a substituent moves from one part of a π-bonded system to another part in an intramolecular reaction with simultaneous rearrangement of the π system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid catalysis is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the ,3Cope rearrangement, Claisen rearrangement, Carroll rearrangement, and the Fischer indole synthesis. Overview of sigmatropic shifts Woodward–Hoffman sigmatropic shift nomencla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of particles in matter. Thermal radiation is generated when heat from the movement of charges in the material (electrons and protons in common forms of matter) is converted to electromagnetic radiation. All matter with a temperature greater than absolute zero emits thermal radiation. At room temperature, most of the emission is in the infrared (IR) spectrum. Particle motion results in charge-acceleration or dipole oscillation which produces electromagnetic radiation. Infrared radiation emitted by animals (detectable with an infrared camera) and cosmic microwave background radiation are examples of thermal radiation. If a radiation object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation. Planck's law describes the spectrum of blackbody radiation, which depends solely on the object's temperature. Wien's displacement law de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suprafacial Antarafacial -1,5-
Antarafacial ( Woodward-Hoffmann symbol a) and suprafacial (s) are two topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or bond breaking processes in or around a reaction center. The reaction center can be a p- or sp''n-''orbital (Woodward-Hoffmann symbol ω), a conjugated system (π) or even a sigma bond (σ). * The relationship is ''antarafacial'' when opposite faces of the π system or isolated orbital are involved in the process (think ''anti''). For a σ bond, it corresponds to involvement of one "interior" lobe and one "exterior" lobe of the bond. * The relationship is ''suprafacial'' when the same face of the π system or isolated orbital are involved in the process (think ''syn''). For a σ bond, it corresponds to involvement of two "interior" lobes or two "exterior" lobes of the bond. The components of all pericyclic reactions, including sigmatropic reactions and cycloadditions, and electrocyclizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roald Hoffmann
Roald Hoffmann (born Roald Safran; July 18, 1937) is a Polish Americans, Polish-American theoretical chemistry, theoretical chemist who won the 1981 Nobel Prize in Chemistry. He has also published plays and poetry. He is the Frank H. T. Rhodes Professor of Humane Letters, Emeritus, at Cornell University, in Ithaca, New York. Early life Escape from the Holocaust Hoffmann was born in Złoczów, Second Polish Republic (now Zolochiv, Ukraine), to a Polish-Jewish family, and was named in honor of the Norway, Norwegian explorer Roald Amundsen. His parents were Clara (Rosen), a teacher, and Hillel Safran, a civil engineer. After Germany invaded Poland and occupied the town, his family was placed in a labor camp where his father, who was familiar with much of the local infrastructure, was a valued prisoner. As the situation grew more dangerous, with prisoners being transferred to extermination camps, the family bribed guards to allow an escape. They arranged with a Ukrainian neighbor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Burns Woodward
Robert Burns Woodward (April 10, 1917 – July 8, 1979) was an American organic chemist. He is considered by many to be the most preeminent synthetic organic chemist of the twentieth century, having made many key contributions to the subject, especially in the synthesis of complex natural products and the determination of their molecular structure. He also worked closely with Roald Hoffmann on theoretical studies of chemical reactions. He was awarded the Nobel Prize in Chemistry in 1965. Early life and education Woodward was born in Boston, Massachusetts, on April 10, 1917. He was the son of Margaret Burns (an immigrant from Scotland who claimed to be a descendant of the poet, Robert Burns) and her husband, Arthur Chester Woodward, himself the son of Roxbury apothecary, Harlow Elliot Woodward. His father was one of the many victims of the 1918 influenza pandemic of 1918. From a very early age, Woodward was attracted to and engaged in private study of chemistry while he att ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antarafacial And Suprafacial
Antarafacial (Woodward–Hoffmann rules, Woodward-Hoffmann symbol a) and suprafacial (s) are two topology, topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or bond breaking processes in or around a reaction center. The reaction center can be a p-orbital, p- or Hybrid orbital, sp''n-''orbital (Woodward-Hoffmann symbol ω), a conjugated system (π) or even a sigma bond (σ). * The relationship is ''antarafacial'' when opposite faces of the π system or isolated orbital are involved in the process (think ''anti''). For a σ bond, it corresponds to involvement of one "interior" lobe and one "exterior" lobe of the bond. * The relationship is ''suprafacial'' when the same face of the π system or isolated orbital are involved in the process (think ''syn''). For a σ bond, it corresponds to involvement of two "interior" lobes or two "exterior" lobes of the bond. The components of all pericyclic reactions, including sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
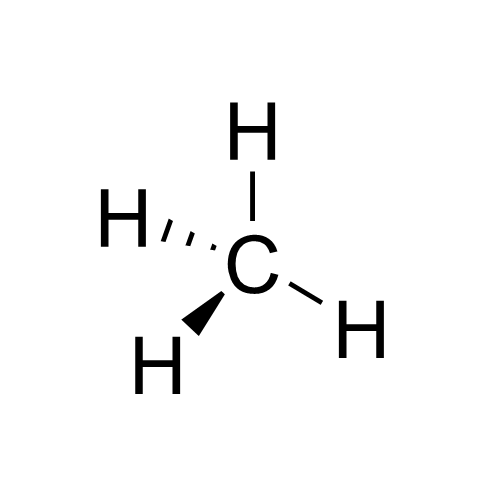
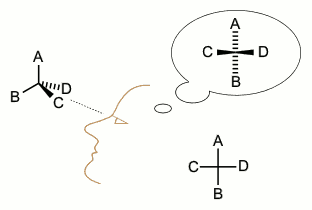
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question ( dynamic stereochemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry Rentention Inversion
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question (dynamic stereochemistry) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Orbital
In atomic theory and quantum mechanics, an atomic orbital is a function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term ''atomic orbital'' may also refer to the physical region or space where the electron can be calculated to be present, as predicted by the particular mathematical form of the orbital. Each orbital in an atom is characterized by a set of values of the three quantum numbers , , and , which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (magnetic quantum number). Alternative to the magnetic quantum number, the orbitals are often labeled by the associated harmonic polynomials (e.g., ''xy'', ). Each such orbital can be occupied by a maximum of two electrons, each with its own projection of spin m_s. The simple names s orbital, p orb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigmatropic Rearrangements
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular reaction. The name ''sigmatropic'' is the result of a compounding of the long-established sigma designation from single carbon–carbon bonds and the Greek word ''tropos'', meaning turn. In this type of rearrangement reaction, a substituent moves from one part of a π-bonded system to another part in an intramolecular reaction with simultaneous rearrangement of the π system. True sigmatropic reactions are usually uncatalyzed, although Lewis acid catalysis is possible. Sigmatropic reactions often have transition-metal catalysts that form intermediates in analogous reactions. The most well-known of the sigmatropic rearrangements are the ,3Cope rearrangement, Claisen rearrangement, Carroll rearrangement, and the Fischer indole synthesis. Overview of sigmatropic shifts Woodward–Hoffman sigmatropic shift nomenclatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |