|
α-Zearalenol
α-Zearalenol is a nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in '' Fusarium spp''. It is the α epimer of β-zearalenol and along with β-zearalenol is a major metabolite of zearalenone formed mainly in the liver but also to a lesser extent in the intestines during first-pass metabolism. A relatively low proportion of β-zearalenol is formed from zearalenone compared to α-zearalenol in humans. α-Zearalenol is about 3- to 4-fold more potent as an estrogen relative to zearalenone. See also * Taleranol Taleranol (INN, USAN) (developmental code name P-1560), or teranol, also known as β-zearalanol, is a synthetic, nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in '' Fusarium spp'' which was never mar ... (β-zearalanol) * Zeranol (α-zearalanol) * Zearalanone References {{DEFAULTSORT:Zearalenol, alpha- Lactones Mycoestrogens Mycotoxins Resorcinols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zearalanone
Zearalanone (ZAN) is a semi-synthetic mycoestrogen that is a derivative of zearalenone (ZEN). Zearalanone is extracted from medical herbs and edible herbs alone with other substance called aflatoxins in the same time by a specific immunoaffinity column. Zearalanone has six analog. They are ZEN, zearalanone, α-zeralanol, β-zeralanol, α-zearalenol, and β-zearalenol. See also * α-Zearalenol * β-Zearalenol * Taleranol Taleranol (INN, USAN) (developmental code name P-1560), or teranol, also known as β-zearalanol, is a synthetic, nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in '' Fusarium spp'' which was never mar ... * Zeranol References Estrogens Lactones Resorcinols {{biochem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeranol
Zeranol (, , ) (brand names Frideron, Ralabol, Ralgro, Ralone, Zerano; developmental code names MK-188, P-1496), or zearanol, also known as α-zearalanol or simply zearalanol, is a synthetic nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in fungi in the ''Fusarium'' genus and is used mainly as an anabolic agent in veterinary medicine. Zeranol is approved for use as a growth promoter in livestock, including beef cattle, under the brand name Ralgro (by Merck Animal Health) in the United States. In Canada, it is approved for use in beef cattle only. Its application is not approved for use in the European Union. However, it is marketed under the brand name Ralone in Spain. Although zeranol may increase cancer cell proliferation in already existing breast cancer, dietary exposure from the use of zeranol-containing implants in cattle is insignificant. Zeranol may be found as a contaminant in fungus-infected crops. It is 3 to 4 times more pote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taleranol
Taleranol (INN, USAN) (developmental code name P-1560), or teranol, also known as β-zearalanol, is a synthetic, nonsteroidal estrogen of the resorcylic acid lactone group related to mycoestrogens found in '' Fusarium spp'' which was never marketed. It is the β epimer of zeranol (α-zearalanol) and is a major metabolite of zeranol but with less biological activity. See also * α-Zearalenol * β-Zearalenol * Zearalanone * Zearalenone Zearalenone (ZEN), also known as RAL and F-2 mycotoxin, is a potent estrogenic metabolite produced by some ''Fusarium'' and '' Gibberella'' species. Specifically, the ''Gibberella zeae ,'' the fungal species where zearalenone was initially detected ... References Lactones Mycoestrogens Mycotoxins Resorcinols {{genito-urinary-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zearalenone
Zearalenone (ZEN), also known as RAL and F-2 mycotoxin, is a potent estrogenic metabolite produced by some ''Fusarium'' and '' Gibberella'' species. Specifically, the ''Gibberella zeae ,'' the fungal species where zearalenone was initially detected, in its asexual/anamorph stage is known as ''Fusarium graminearum.'' Several ''Fusarium'' species produce toxic substances of considerable concern to livestock and poultry producers, namely deoxynivalenol, T-2 toxin, HT-2 toxin, diacetoxyscirpenol (DAS) and zearalenone. Particularly, ZEN is produced by '' Fusarium graminearum'', ''Fusarium culmorum'', '' Fusarium cerealis'', ''Fusarium equiseti'', '' Fusarium verticillioides'', and ''Fusarium incarnatum''. Zearalenone is the primary toxin that binds to estrogen receptors, causing infertility, abortion or other breeding problems, especially in swine. Often, ZEN is detected together with deoxynivalenol in contaminated samples and its toxicity needs to be considered in combination with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative. Nonsteroidal anti-inflammatory drugs (NSAIDs) are distinguished from corticosteroids as a class of anti-inflammatory agents. List of nonsteroidal steroid receptor modulators Examples include the following: * Estrogens: benzestrol, bifluranol, estrobin (DBE), diethylstilbestrol (stilbestrol), dienestrol, erteberel, fosfestrol, hexestrol (dihydroxystilbestrol), methallenestril, methestrol, methestrol dipropionate, paroxypropione, prinaberel, and triphenylethylene, as well as many xenoestrogens * : acolbifene, afimoxifene, arzoxifene, bazedoxifene, broparestrol, chlorotrianisene, clomifene, clomifenoxide, cyclofenil, droloxifene, enclomifene, endoxifen, ethamoxytriphetol, fispemifene, idoxifene, lasofoxifene, levormeloxifene, miproxifene, nafoxidine, nitromifene, ormeloxifene, ospemifene, panomifene, pipendoxifene, raloxifene, tamoxifen, toremifene, trioxifene, zindoxifene, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
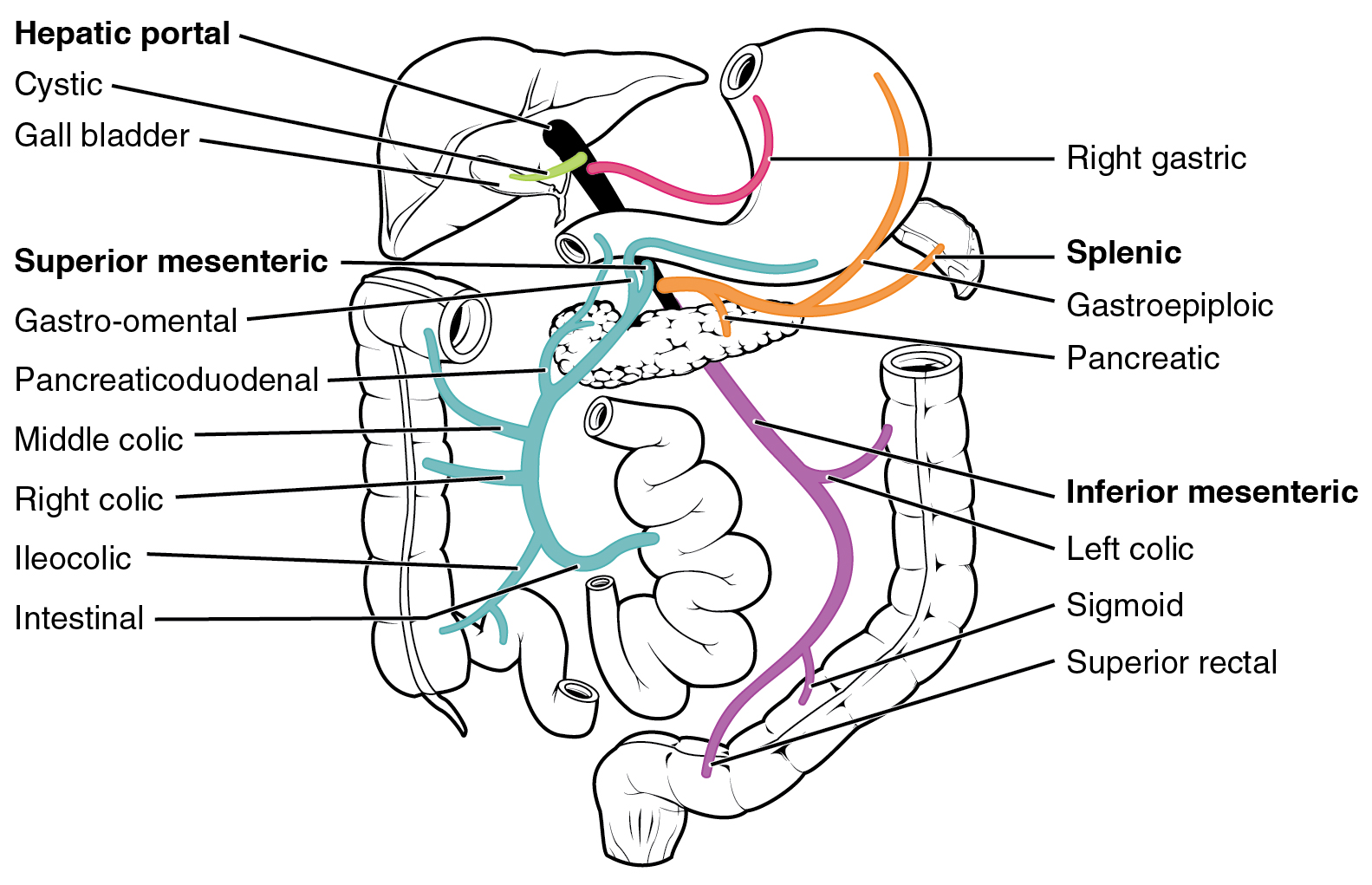
Intestine
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organs of the digestive system, in humans and other animals, including the esophagus, stomach, and intestines. Food taken in through the mouth is digested to extract nutrients and absorb energy, and the waste expelled at the anus as feces. ''Gastrointestinal'' is an adjective meaning of or pertaining to the stomach and intestines. Most animals have a "through-gut" or complete digestive tract. Exceptions are more primitive ones: sponges have small pores (ostia) throughout their body for digestion and a larger dorsal pore ( osculum) for excretion, comb jellies have both a ventral mouth and dorsal anal pores, while cnidarians and acoels have a single pore for both digestion and excretion. The human gastrointestinal tract consists of the esophagus, stomach, and intestines, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycoestrogens
Mycoestrogens are xenoestrogens produced by fungi. They are sometimes referred to as mycotoxins. Among important mycoestrogens are zearalenone, zearalenol and zearalanol. Although all of these can be produced by various ''Fusarium'' species, zearalenol and zearalanol may also be produced endogenously in ruminants that have ingested zearalenone. Alpha-zearalanol is also produced semisynthetically, for veterinary use; such use is prohibited in the European Union. Mechanism of action Mycoestrogens act as agonists of the estrogen receptors, ERα and ERβ. Sources Mycoestrogens are produced by various strains of fungi, many of which fall under the genus ''Fusarium''. ''Fusarium'' fungi are filamentous fungi that are found in the soil and are associated with plants and some crops, especially cereals. Zearalenone is mainly produced by ''F. graminearum'' and ''F. culmorum'' strains, which inhabit different areas depending on temperature and humidity. ''F. graminearum'' prefers to inha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactones
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring. Lactones are formed by intramolecular esterification of the corresponding hydroxycarboxylic acids, which takes place spontaneously when the ring that is formed is five- or six-membered. Lactones with three- or four-membered rings (α-lactones and β-lactones) are very reactive, making their isolation difficult. Special methods are normally required for the laboratory synthesis of small-ring lactones as well as those that contain rings larger than six-membered. Nomenclature Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First-pass Metabolism
The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism whereby the concentration of a drug, specifically when administered orally, is greatly reduced before it reaches the systemic circulation. It is the fraction of drug lost during the process of absorption which is generally related to the liver and gut wall. Notable drugs that experience a significant first-pass effect are buprenorphine, chlorpromazine, cimetidine, diazepam, ethanol (drinking alcohol), imipramine, insulin, lidocaine, midazolam, morphine, pethidine, propranolol, and tetrahydrocannabinol (THC). First pass metabolism may occur in the liver (for propranolol, lidocaine, clomethiazole, and NTG) or in the gut (for benzylpenicillin and insulin). After a drug is swallowed, it is absorbed by the digestive system and enters the hepatic portal system. It is carried through the portal vein into the liver before it reaches the rest of the body. The liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it is located in the right upper quadrant of the abdomen, below the diaphragm. Its other roles in metabolism include the regulation of glycogen storage, decomposition of red blood cells, and the production of hormones. The liver is an accessory digestive organ that produces bile, an alkaline fluid containing cholesterol and bile acids, which helps the breakdown of fat. The gallbladder, a small pouch that sits just under the liver, stores bile produced by the liver which is later moved to the small intestine to complete digestion. The liver's highly specialized tissue, consisting mostly of hepatocytes, regulates a wide variety of high-volume biochemical reactions, including the synthesis and breakdown of small and complex molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen
Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy. Estrogens are synthesized in all vertebrates and some insects. Their presence in both vertebrates and insects suggests that estrogenic sex hormones have an ancient evolutionary history. Quantitatively, estrogens circulate at lower levels than androgens in both men and women. While estrogen levels are significantly lower in males than in females, estrogens nevertheless have important physiological roles in males. Like all steroid hormones, estrogens readily diffuse across the cell membrane. Once inside the cell, they bind to and activate estrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, catalytic activity of their own (usually as a cofactor to an enzyme), defense, and interactions with other organisms (e.g. pigments, odorants, and pheromones). A primary metabolite is directly involved in normal "growth", development, and reproduction. Ethylene exemplifies a primary metabolite produced large-scale by industrial microbiology. A secondary metabolite is not directly involved in those processes, but usually has an important ecological function. Examples include antibiotics and pigments such as resins and terpenes etc. Some antibiotics use primary metabolites as precursors, such as actinomycin, which is created from the primary metabolite tryptophan. Some sugars are metabolites, such as fructose or glucose, which ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |