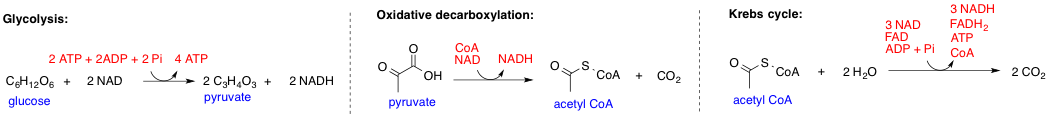|
δ13C
In geochemistry, paleoclimatology, and paleoceanography ''δ''13C (pronounced "delta c thirteen") is an isotopic signature, a measure of the ratio of stable isotopes 13C : 12C, reported in parts per thousand (per mil, ‰). The measure is also widely used in archaeology for the reconstruction of past diets, particularly to see if marine foods or certain types of plants were consumed The definition is, in per mil: :\delta \ce = \left( \frac - 1 \right) \times 1000 ‰ where the standard is an established reference material. ''δ''13C varies in time as a function of productivity, the signature of the inorganic source, organic carbon burial, and vegetation type. Biological processes preferentially take up the lower mass isotope through kinetic fractionation. However some abiotic processes do the same, methane from hydrothermal vents can be depleted by up to 50%. Reference standard The standard established for carbon-13 work was the Pee Dee Belemnite (PDB) and was based on a Cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis. Stable isotopes The atomic mass of different isotopes affect their chemical kinetic behavior, leading to natural isotope separation processes. Carbon isotopes For example, different sources and sinks of methane have different affinity for the 12C and 13C isotopes, which allows distinguishing between different sources by the 13C/12C ratio in methane in the air. In geochemistry, paleoclimatology and paleoceanography this ratio is called δ13C. The ratio is calculated with respect to Pee Dee Belemnite (PDB) standard: :\delta \ce_\mathrm = \left(\frac - 1\right) \cdot 1000 ‰ Similarly, carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopic Signature
An isotopic signature (also isotopic fingerprint) is a ratio of non-radiogenic ' stable isotopes', stable radiogenic isotopes, or unstable radioactive isotopes of particular elements in an investigated material. The ratios of isotopes in a sample material are measured by isotope-ratio mass spectrometry against an isotopic reference material. This process is called isotope analysis. Stable isotopes The atomic mass of different isotopes affect their chemical kinetic behavior, leading to natural isotope separation processes. Carbon isotopes For example, different sources and sinks of methane have different affinity for the 12C and 13C isotopes, which allows distinguishing between different sources by the 13C/12C ratio in methane in the air. In geochemistry, paleoclimatology and paleoceanography this ratio is called δ13C. The ratio is calculated with respect to Pee Dee Belemnite (PDB) standard: :\delta \ce_\mathrm = \left(\frac - 1\right) \cdot 1000 ‰ Similarly, carbon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope Geochemistry
Isotope geochemistry is an aspect of geology based upon the study of natural variations in the relative abundances of isotopes of various elements. Variations in isotopic abundance are measured by isotope ratio mass spectrometry, and can reveal information about the ages and origins of rock, air or water bodies, or processes of mixing between them. Stable isotope geochemistry is largely concerned with isotopic variations arising from mass-dependent isotope fractionation, whereas radiogenic isotope geochemistry is concerned with the products of natural radioactivity. Stable isotope geochemistry For most stable isotopes, the magnitude of fractionation from kinetic and equilibrium fractionation is very small; for this reason, enrichments are typically reported in "per mil" (‰, parts per thousand). These enrichments (δ) represent the ratio of heavy isotope to light isotope in the sample over the ratio of a standard. That is, :\delta \ce = \left( \frac -1 \right) \times 1000 â ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-13
Carbon-13 (13C) is a natural, stable isotope of carbon with a nucleus containing six protons and seven neutrons. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on Earth. Detection by mass spectrometry A mass spectrum of an organic compound will usually contain a small peak of one mass unit greater than the apparent molecular ion peak (M) of the whole molecule. This is known as the M+1 peak and comes from the few molecules that contain a 13C atom in place of a 12C. A molecule containing one carbon atom will be expected to have an M+1 peak of approximately 1.1% of the size of the M peak, as 1.1% of the molecules will have a 13C rather than a 12C. Similarly, a molecule containing two carbon atoms will be expected to have an M+1 peak of approximately 2.2% of the size of the M peak, as there is double the previous likelihood that any molecule will contain a 13C atom. In the above, the mathematics and chemistry have been simplified, however it can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reference Materials For Stable Isotope Analysis
Isotopic reference materials are compounds (solids, liquids, gasses) with well-defined isotopic compositions and are the ultimate sources of accuracy in mass spectrometric measurements of isotope ratios. Isotopic references are used because mass spectrometers are highly fractionating. As a result, the isotopic ratio that the instrument measures can be very different from that in the sample's measurement. Moreover, the degree of instrument fractionation changes during measurement, often on a timescale shorter than the measurement's duration, and can depend on the characteristics of the sample itself. By measuring a material of known isotopic composition, fractionation within the mass spectrometer can be removed during post-measurement data processing. Without isotope references, measurements by mass spectrometry would be much less accurate and could not be used in comparisons across different analytical facilities. Due to their critical role in measuring isotope ratios, and in pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane Clathrate
Methane clathrate (CH4·5.75H2O) or (8CH4·46H2O), also called methane hydrate, hydromethane, methane ice, fire ice, natural gas hydrate, or gas hydrate, is a solid clathrate compound (more specifically, a clathrate hydrate) in which a large amount of methane is trapped within a crystal structure of water, forming a solid similar to ice. Originally thought to occur only in the outer regions of the Solar System, where temperatures are low and water ice is common, significant deposits of methane clathrate have been found under sediments on the ocean floors of the Earth. Methane hydrate is formed when hydrogen-bonded water and methane gas come into contact at high pressures and low temperatures in oceans. Methane clathrates are common constituents of the shallow marine geosphere and they occur in deep Sedimentary rock, sedimentary structures and form outcrops on the ocean floor. Methane hydrates are believed to form by the precipitation or crystallisation of methane migrating from d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paleocene–Eocene Thermal Maximum
The Paleocene–Eocene thermal maximum (PETM), alternatively (ETM1), and formerly known as the "Initial Eocene" or "", was a time period with a more than 5–8 °C global average temperature rise across the event. This climate event occurred at the time boundary of the Paleocene and Eocene geological epochs. The exact age and duration of the event is uncertain but it is estimated to have occurred around 55.5 million years ago. The associated period of massive carbon release into the atmosphere has been estimated to have lasted from 20,000 to 50,000 years. The entire warm period lasted for about 200,000 years. Global temperatures increased by 5–8 °C. The onset of the Paleocene–Eocene thermal maximum has been linked to volcanism and uplift associated with the North Atlantic Igneous Province, causing extreme changes in Earth's carbon cycle and a significant temperature rise. The period is marked by a prominent negative excursion in carbon stable isotope () reco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell. These enzymes often require dietary minerals, vitamins, and other cofactors to function. Different metabolic pathways function based on the position within a eukaryotic cell and the significance of the pathway in the given compartment of the cell. For instance, the, electron transport chain, and oxidative phosphorylation all take place in the mitochondrial membrane. In contrast, glycolysis, pentose phosphate pathway, and fatty acid biosynthesis all occur in the cytosol of a cell. There are two types of metabolic pathways that are character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geology (journal)
''Geology'' is a peer-reviewed publication of the Geological Society of America (GSA). GSA says (ISSN 0091-7613) that it is the most widely read scientific journal in the field of earth science. It is published monthly, with each issue containing 20 or more articles. One of the goals of the journal is to provide a forum for shorter articles (four pages each) and less focus on purely academic research–type articles. According to the ''Journal Citation Reports'', the journal had a 2020 impact factor of 5.399. The editorial board is very diverse. The journal is indexed in Scopus and SCImago. See also *List of scientific journals *List of scientific journals in earth and atmospheric sciences A ''list'' is any set of items in a row. List or lists may also refer to: People * List (surname) Organizations * List College, an undergraduate division of the Jewish Theological Seminary of America * SC Germania List, German rugby union ... References External links * [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Productivity
In ecology, primary production is the synthesis of organic compounds from atmospheric or aqueous carbon dioxide. It principally occurs through the process of photosynthesis, which uses light as its source of energy, but it also occurs through chemosynthesis, which uses the oxidation or reduction of inorganic chemical compounds as its source of energy. Almost all life on Earth relies directly or indirectly on primary production. The organisms responsible for primary production are known as ''primary producers'' or autotrophs, and form the base of the food chain. In terrestrial ecoregions, these are mainly plants, while in aquatic ecoregions algae predominate in this role. Ecologists distinguish primary production as either ''net'' or ''gross'', the former accounting for losses to processes such as cellular respiration, the latter not. Overview Primary production is the production of chemical energy in organic compounds by living organisms. The main source of this energy is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C4 Carbon Fixation
carbon fixation or the Hatch–Slack pathway is one of three known photosynthetic processes of carbon fixation in plants. It owes the names to the 1960's discovery by Marshall Davidson Hatch and Charles Roger Slack that some plants, when supplied with 14, incorporate the 14C label into four-carbon molecules first. fixation is an addition to the ancestral and more common carbon fixation. The main carboxylating enzyme in photosynthesis is called RuBisCO, which catalyses two distinct reactions using either (carboxylation) or oxygen (oxygenation) as a substrate. The latter process, oxygenation, gives rise to the wasteful process of photorespiration. photosynthesis reduces photorespiration by concentrating around RuBisCO. To ensure that RuBisCO works in an environment where there is a lot of carbon dioxide and very little oxygen, leaves generally differentiate two partially isolated compartments called mesophyll cells and bundle-sheath cells. is initially fixed in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C3 Carbon Fixation
carbon fixation is the most common of three metabolic pathways for carbon fixation in photosynthesis, along with and CAM. This process converts carbon dioxide and ribulose bisphosphate (RuBP, a 5-carbon sugar) into two molecules of 3-phosphoglycerate through the following reaction: :CO2 + H2O + RuBP → (2) 3-phosphoglycerate This reaction was first discovered by Melvin Calvin, Andrew Benson and James Bassham in 1950. C3 carbon fixation occurs in all plants as the first step of the Calvin–Benson cycle. (In and CAM plants, carbon dioxide is drawn out of malate and into this reaction rather than directly from the air.) Plants that survive solely on fixation ( plants) tend to thrive in areas where sunlight intensity is moderate, temperatures are moderate, carbon dioxide concentrations are around 200 ppm or higher, and groundwater is plentiful. The plants, originating during Mesozoic and Paleozoic eras, predate the plants and still represent approximately 95% of Eart ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |