|
Xyloside
A xyloside is a type of glycoside derived from the sugar xylose. Proteoglycan (PG) synthesis is initiated by the transfer of D-xylose from UDP-xylose to a serine residue in core proteins. This natural primer acts as a template for the assembly of heparin sulfate, heparin, chondroitin sulfate, and dermatan sulfate side chains, depending on the tissue. However, in 1973 it was determined that synthetic B-D-xylosides can prime glycosaminoglycan (GAG) synthesis by substituting for the core xylosylated protein. Many Beta-D-xylosides have been studied for use as xylose primes with varying results. # Priming requires the Beta-anomer of xylose. # Priming activity correlates with the activity of the aglycone (cite). # The most active xyloside primers contain O or S in glycosidic linkage. # Priming is dose dependent.Lugemwa and Esko 1991 # Beta-D-xylosides prime GAGs in most cells. # Most of the material created from Beta-D-xylosides priming is excreted into the growth media. # Beta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a '' C-glycoside'') glycosidic bond. According to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylose
Xylose ( grc, ξύλον, , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is derived from hemicellulose, one of the main constituents of biomass. Like most sugars, it can adopt several structures depending on conditions. With its free aldehyde group, it is a reducing sugar. Structure The acyclic form of xylose has chemical formula . The cyclic hemiacetal isomers are more prevalent in solution and are of two types: the pyranoses, which feature six-membered rings, and the furanoses, which feature five-membered rings (with a pendant group). Each of these rings is subject to further isomerism, depending on the relative orientation of the anomeric hydroxy group. The dextrorotary form, -xylose, is the one that usually occurs endogenously in living things. A levorotary form, -xylose, can be synthesize ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich proteoglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
D-xylose
Xylose ( grc, ξύλον, , "wood") is a sugar first isolated from wood, and named for it. Xylose is classified as a monosaccharide of the aldopentose type, which means that it contains five carbon atoms and includes an aldehyde functional group. It is derived from hemicellulose, one of the main constituents of biomass. Like most sugars, it can adopt several structures depending on conditions. With its free aldehyde group, it is a reducing sugar. Structure The acyclic form of xylose has chemical formula . The cyclic hemiacetal isomers are more prevalent in solution and are of two types: the pyranoses, which feature six-membered rings, and the furanoses, which feature five-membered rings (with a pendant group). Each of these rings is subject to further isomerism, depending on the relative orientation of the anomeric hydroxy group. The dextrorotary form, -xylose, is the one that usually occurs endogenously in living things. A levorotary form, -xylose, can be synthesize ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heparin Sulfate
Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan (HSPG, i.e. Heparan Sulfate ProteoGlycan) in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. It is in this form that HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB (Granzyme B), and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2. Proteoglycans The major cell membrane HSPGs are the transmembrane syndecans and the glycosylphosphatidylinositol (GPI) anchored glypicans. Other minor forms of membrane HSPG include betaglycan and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
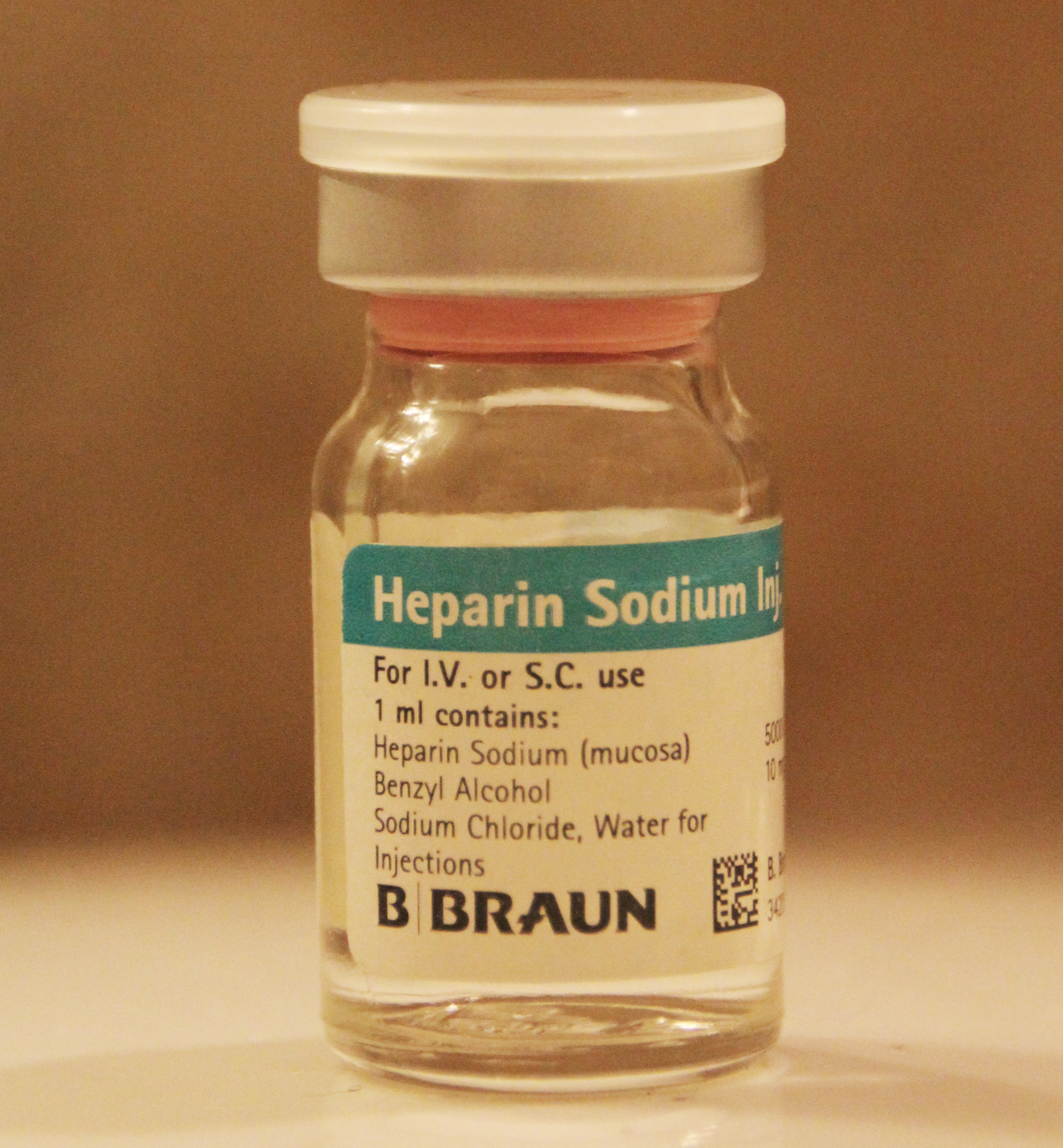
Heparin
Heparin, also known as unfractionated heparin (UFH), is a medication and naturally occurring glycosaminoglycan. Since heparins depend on the activity of antithrombin, they are considered anticoagulants. Specifically it is also used in the treatment of heart attacks and unstable angina. It is given intravenously or by injection under the skin. Other uses for its anticoagulant properties include inside blood specimen test tubes and kidney dialysis machines. Common side effects include bleeding, pain at the injection site, and low blood platelets. Serious side effects include heparin-induced thrombocytopenia. Greater care is needed in those with poor kidney function. Heparin is contraindicated for suspected cases of vaccine-induced pro-thrombotic immune thrombocytopenia (VIPIT) secondary to SARS-CoV-2 vaccination, as heparin may further increase the risk of bleeding in an anti-PF4/heparin complex autoimmune manner, in favor of alternative anticoagulant medications (such as arg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chondroitin Sulfate
Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars ( N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Chondroitin sulfate is an important structural component of cartilage, and provides much of its resistance to compression. Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis, although large clinical trials failed to demonstrate any symptomatic benefit of chondroitin. Medical use Chondroitin is used in dietary supplements as an alternative medicine to treat osteoarthritis. It is also approved and regulated as a symptomatic slow-acting drug for this disease (SYSADOA) in Europe and some other countries. It is commonly sold together with glucosamine. A 2015 Cochrane review of clini ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dermatan Sulfate
Dermatan sulfate is a glycosaminoglycan (formerly called a mucopolysaccharide) found mostly in skin, but also in blood vessels, heart valves, tendons, and lungs. It is also referred to as chondroitin sulfate B, although it is no longer classified as a form of chondroitin sulfate by most sources. The formula is C14H21NO15S. This carbohydrate is composed of linear polymers of disaccharide units that contain, N-acetyl galactosamine (GalNAc) and iduronic acid (IdoA). These repeating units are sulfated at a variety of positions. Dermatan sulfate is a component of the compound sulodexide. Function Dermatan sulfate may have roles in coagulation, cardiovascular disease, carcinogenesis, infection, wound repair, maintains the shape of galactosamine 4-sulfate skin and fibrosis. Pathology Dermatan sulfate accumulates abnormally in several of the mucopolysaccharidosis disorders. An excess of dermatan sulfate in the mitral valve is characteristic of myxomatous degeneration of the leaflet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosaminoglycan
Glycosaminoglycans (GAGs) or mucopolysaccharides are long, linear polysaccharides consisting of repeating disaccharide units (i.e. two-sugar units). The repeating two-sugar unit consists of a uronic sugar and an amino sugar, except in the case of the sulfated glycosaminoglycan keratan, where, in place of the uronic sugar there is a galactose unit. GAGs are found in vertebrates, invertebrates and bacteria. Because GAGs are highly polar molecules and attract water; the body uses them as lubricants or shock absorbers. Mucopolysaccharidoses are a group of metabolic disorders in which abnormal accumulations of glycosaminoglycans occur due to enzyme deficiencies. Production Glycosaminoglycans vary greatly in molecular mass, disaccharide structure, and sulfation. This is because GAG synthesis is not template driven, as are proteins or nucleic acids, but constantly altered by processing enzymes. GAGs are classified into four groups, based on their core disaccharide structures. Hepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
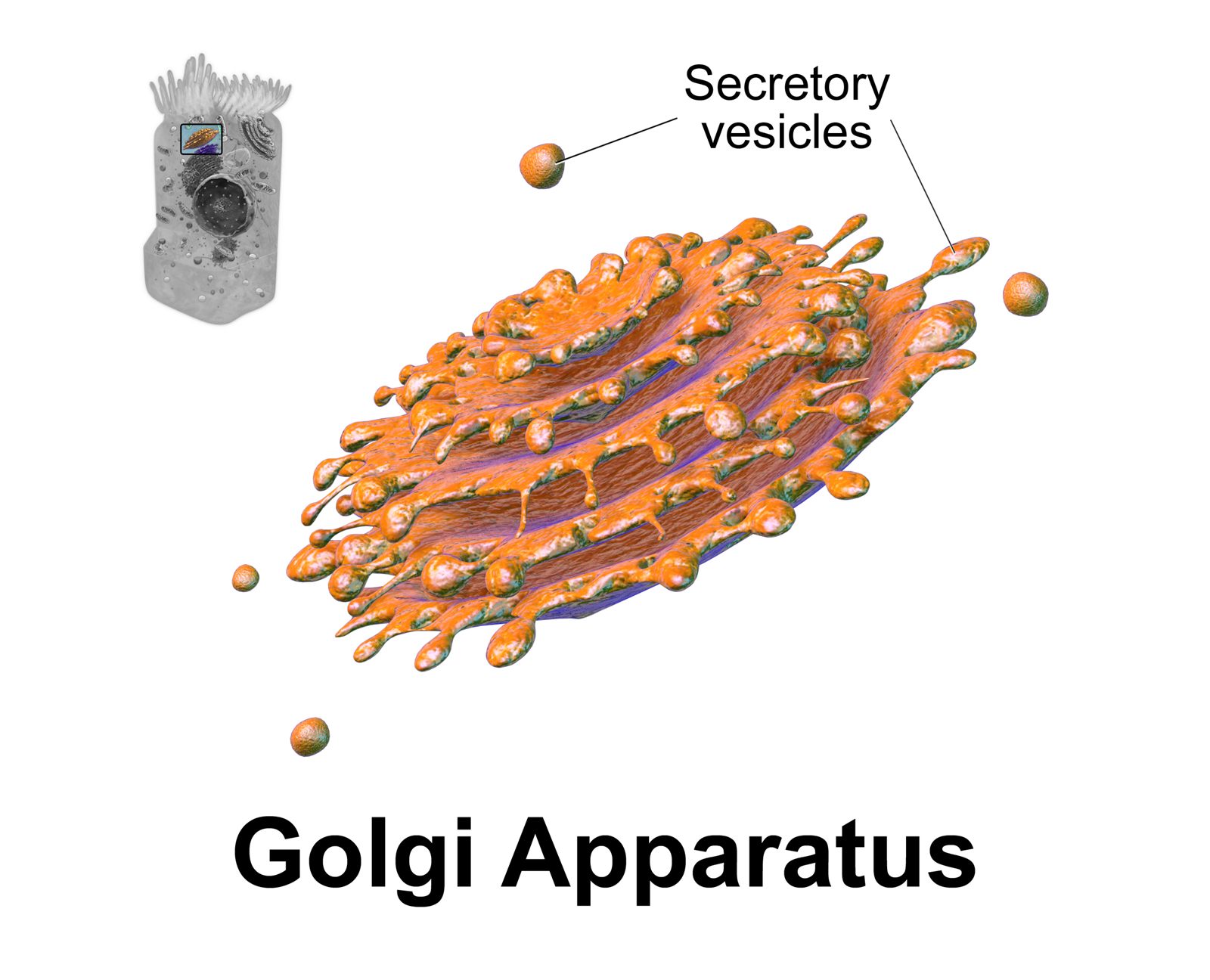
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |